Nutraceutical, Antioxidant and Hepatic Histomorphological Effects of Tetrapleura tetraptera Leaves in Monosodium Glutamate-intoxicated Rats
| Received 02 Sep, 2020 |
Accepted 21 Oct, 2020 |
Published 15 Nov, 2020 |
Background and Objectives: Tetrapleura tetraptera, a widely utilized plant in West Africa with high nutritious potential, has been implicated in amelioration diseases and conditions such as arthritis, asthma, diabetes mellitus, hypertension, epilepsy, schistosomiasis and even prevention of post-partum contraction. Monosodium glutamate on its own is consumed widely and does not appear in labels hence giving room for overdose. The objective of this study is to determine the nutraceutical composition, serum bio-functional parameters and serum antioxidant activities of Tetrapleura tetraptera in monosodium glutamate-intoxicated rats. Materials and Methods: Neutraceutical composition and histo-morphological effects of Tetrapleura tetraptera leave in Monosodium Glutamate-intoxicated rats were determined by using standard protocols. One way ANOVA was used in the statistical analysis using SPSS package version 22. Results: The result of the mineral content (mg/100g) was in the order sodium> magnesium> calcium> potassium while that of the vitamins was B3>E>B2>B1>A. The highest proximate (%) content was carbohydrates while the lowest was fiber (1.02±0.01). The extract have very high phenolic (823.07-10.62 mg 100 g-1) and flavonoid (672.66±5.07 mg 100 g-1). The extract also showed very high FRAP and DPPH activities. There was a significant (p<0.05) decrease in the alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase activity of the group's co-treated monosodium glutamate (MSG) and different concentrations of Tetrapleura tetraptera with the highest decrease noticed in low dose group that received only 200 mg kg-1. This same trend was noticed in GSH concentration. Conclusion: Conclusively, Tetrapleura tetraptera, have high antioxidant activity, high mineral composition and ameliorates the toxic assault on the liver and kidney as established on monosodium glutamate-intoxicated rats.
INTRODUCTION
Plants are rich sources of bioactive components that have desirable health benefits and are traditionally known to be used for the prevention of chronic diseases1. Food security, health and the socio-economic welfare of both rural and urban communities have been sustained through non-timber forest products such as fruits, seeds, roots, stems, leaves and flowers. Tetrapleura tetraptera is a widely utilized plant in West Africa for their perceived nutritional and medicinal value2. It is used as a popular seasoning spice in Southern and Eastern Nigeria3 and its fruits is used for the management of convulsions, leprosy, inflammation, rheumatism, flatulence, jaundice and fevers as well as the management and control of adult – onset type 2 diabetes mellitus2. Many reports are available on the use of this plant as a spice and in home remedies for the treatment of many human illnesses3.
Monosodium glutamate (MSG) is one of the world's most extensively used food additive. It is the sodium of the non-essential amino acid glutamates. MSG is frequently added to meals as a flavor enhancer. It is known to have some adverse affects in humans and experimental animals which include the Chinese restaurant syndrome characterized by neuro-excitotoxicity4, and obesity5, nephrotoxicity6, hepatotoxicity7 and induced oxidative stress in hepatic and cardiac tissues in experimental animals8.
Oxidative stress is now recognized to play a role in the pathophysiology of many different disorders including ageing, complications of pregnancy and diseases like cancer, heart disease and Parkinson's disease9.
Antioxidants are capable of stabilizing, or deactivating, free radicals before they damage cells. The body's natural antioxidant defense system comprises endogenous antioxidant enzymes including; glutathione reductase, superoxide dismutase, glutathione peroxidase, and catalase. These enzymes act by catalyzing free radicals thus quenching pro-oxidant reactions. Furthermore, dietary antioxidants such as ascorbic acid (vitamin C), tocopherols and tocotrienols (vitamin E), carotenoids and other low molecular weight compounds, such as glutathione also aid in stabilizing free radicals10,11.
Since MSG is added in food without appearing on labels which increases the possibility of overdose thus hepatic assault; and Tetrapleura tetraptera is a natural edible leaf implicated in ameliorating numerous disease conditions, hence this study aimed at determining the Nutraceutical composition of Tetrapleura Tetraptera Leaves and its effect on hepatic histo-morphology, serum bio-functional parameters and serum antioxioxidant activities in Monosodium Glutamate-intoxicated Rats.
MATERIALS AND METHODS
Study Area: This study was carried out between January, 2019 to May, 2019 at the laboratory unit of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria.
Collection of samples and preparation of extract: Leaves of Tetrapleura tetraptera were collected from Michael Okpara University of Agriculture, Umudike, Abia State Nigeria. The plant sample was identified and authenticated by a Botanist in the Department of Plant Science and Biotechnology, College of Natural Science, Michael Okpara University of Agriculture, Umudike. The leaves were thoroughly washed with tap water to remove soil and other debris that may contaminate the plant sample. The washed sample was then air-dried under shade in postgraduate Laboratory for 28 days and the dried sample was pulverized using Thomas Laboratory Mill (Crypto model, USA,). The resultant Powder sample weighed 500g and was soaked in 2.5 litres of 95% methanol and extracted using filter paper. The crude methanol extract was kept in air-tight container and stored in a refrigerator and hereafter refered to as Tetrapleura tetraptera leaves ethanol extract (TTLEE).
Animals: Sixty adult male albino rats (110-145g) obtained from the animal house unit of the Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria were used. They were acclimatization for 2 weeks and then were randomly divided into six treatment groups of 10 rats each and with each group housed in aluminum cage measuring 20"x15". All animals had access to food and water ad libitum and were maintained under standard laboratory conditions with light and dark cycles of 12h each and room temperature of 25ºC. All guidelines involving the use and care of laboratory animals were duly observed.
Phytochemical composition of Tetrapleura tetraptera: The phytochemical composition of Tetrapleura tetraptera was determined using the method described earlier by AOAC12. However, the carbohydrate content was determined by using the following formula13.
The mineral element composition was determined by the method described by AOAC14 while for the anti-nutrient composition, Hydrocyanide and Oxalate contents were determined following the method of AOAC12. Tannin content was established by Sofowara15. Alkaloid content was ascertained by the method Harbone16. Flavonoid content was determined using the method as by Bohm and Koupai17 while vitamin content was determined by the method as described by Onwuka18.
For the in-vitro anti-oxidant activity of TTLEE, FRAP assay was carried out following the method of Benzie and Strain19 and the DPPH scavenging assay was carried out based on the method described by Brand-Williams et al.20 with slight modification. Different concentrations (31.25-500 μg mL–1) of the extracts and ascorbic acid (standard) were thoroughly mixed with 5 mL of methanolic DPPH solution (33 mg L–1) in test-tubes and the resulting solution was kept standing for 10 minutes at 37°C before the optical density (OD) was measured at 517 nm. The measurement was repeated with three sets and an average of the reading was considered. The percentage radical scavenging activity was calculated from the following formula also described by Brand-Williams et al.20:
Where A0 was the absorbance of the control and A1 was the absorbance in the presence of the samples.
The procedure for determining AST and ALT are similar. The method as described by Reitman and Frankel21 was used. This method is based on the principle that 2,4- Dinitrophenylhydrazone could react with pyruvate hydrazone (ALT) or oxaloacetate hydrazone (AST) to form a colour complex which could be measured photometrically at 564nm. The serum alkaline phosphatase (ALP) was estimated by the endpoint colorimetric method22.
For the determination of in vivo antioxidant activities of TTLEE, malondialdehyde (MDA) concentration was determined according to the method as described by Varshey and Kale23. Catalase activity was assayed using the method described by Aebi24 while glutathione concentration was determined according to the method as described by Habig et al.25.
For the hepatic histo-morphological examination, the method proposed by Clayden26 was used. The excised organs were rinsed in 0.9% saline solution and preserved in 10% formaldehyde solution. It was embedded in paraffin wax and sectioned into 4-6 microns. The sections were stained with hematoxylin and eosin and photographed.
Induction of toxicity: Toxicity was induced using 8000 mg/kg body weight of the monosodium glutamate was orally administered to the rats daily for 14 days according to27 .
Study design for in-vivo antioxidant activity of TTLEE: The 6 groups of experimental animals were treated according to the protocol below:
| Group 1: | 8000 mg/kg body weight of MSG and 200 mg/kg of TTLEE |
| Group 2: | 8000 mg/kg body weight of MSG and 400 mg/kg of TTLEE |
| Group 3: | 8000 mg/kg body weight of MSG and 600 mg/kg of TTLEE |
| Group 4: | Feed and water only and served as the normal control group |
| Group 5: | 200 mg/kg body weight of TTLEE only |
| Group 6: | 8000 mg/kg body weight of mono-sodium glutamate (MSG) only and served as the negative control |
At the end of 14 days of treatment, the animals were sacrificed and blood was collected by cardiac puncture into plane bottles for determination of liver enzymes.
Calculation of diagnostic ratios and change relative to groups: Diagnostic ratios were calculated from the result of corresponding parameters as obtained in this study. The calculation of change relative to any group was developed and used severally. Change relative to either control or MSG- group was calculated using the relationship described by6:
Where K represents the constant group hence constant value and V represent the variable groups hence variable values.
Statistical analysis: Descriptive statistics and test for significance in mean were carried out on the data generated by one-way analysis of variance (ANOVA) with the statistical package for social sciences (SPSS) version 22. The turkey post hoc test were used to identify the means that differ significantly at p<0.05. Results were expressed as mean±standard error of mean SEM
RESULTS
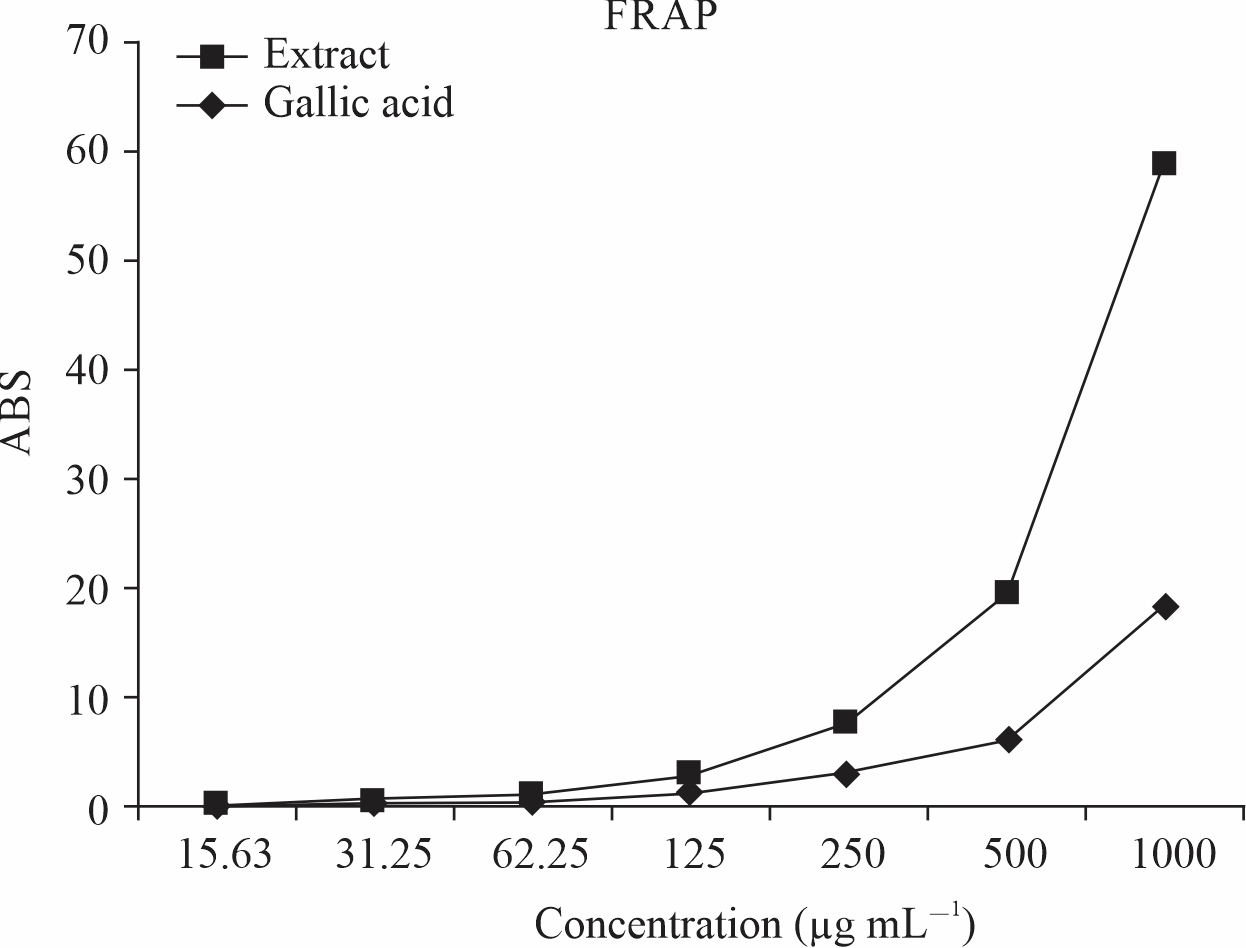
The result as shown in Fig. 1 revealed an increase in reducing the power of Tetrapleura
tetraptera leaves with an increase in concentration. The result showed that the extract
of Tetrapleura tetraptera leaves has stronger reducing power (40.47) as compared to
the standard (gallic acid) (18.35) at the same concentration. This suggested that
Tetrapleura tetraptera leaves have antioxidant properties.
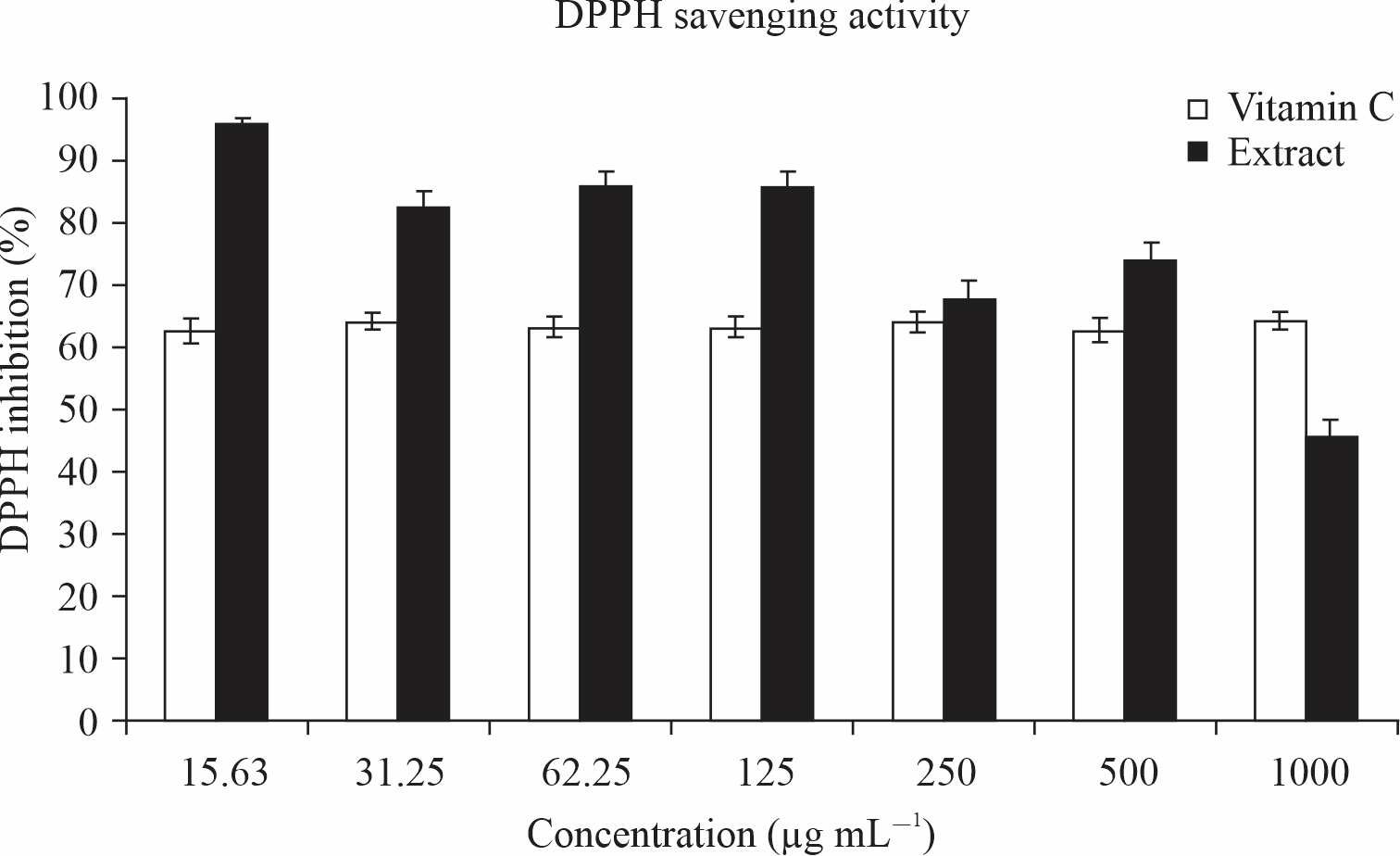
The result as shown in Fig. 2 revealed a significant (p<0.05) higher % of DPPH inhibition by Tetrapleura tetraptera leaves as compared to ascorbic acid (vitamin c) across all concentrations except at 1000 μg ml–1. Tetrapleura tetraptera leaves showed the highest DPPH inhibition at the lowest concentration (15.63 μg ml–1) and the lowest inhibition at 1000 μg ml–1.
 |
|||
Fig. 1: Ferric reducing antioxidant power of Tetrapleura tetraptera leaves |
 |
|||
Fig. 2: DPPH activity of Tetrapleura tetraptera leaves |
The result as shown in Table 1 indicated that Tetrapleura tetraptera has a very high mineral composition with sodium being the highest composite (663.73±1.27 mg 100 mL–1) and potassium the lowest composite (5.70±0.02 mg 100 mL–1).
The result as shown in Table 2 indicated that Tetrapleura tetraptera has high carbohydrates (68.81±0.08) and protein (19.75±0.05) while relatively having very low fiber composition (1.02±0.01).
As shown in Table 3, Tetrapleura tetraptera showed rich vitamin composition with the highest vitamin being vitamins A (315.81±5.83 μg g–1) while the lowest vitamin composition was B3 (21.90±1.21 μg g–1).
The result as shown in Table 4 indicated very high Tetrapleura tetraptera composition of phenolics (823.07±10.62 mg 100 g–1) and flavonoids (672.66±5.07 mg 100 g–1) which could contribute to antioxidant properties. However, Glycosides (2.41±0.12 mg 100 g–1) was its lowest phytochemical composition.
The result as shown in Table 5 indicated a significant (p<0.05) dose-dependent decrease in the AST activity across the co-treated groups when compared to the MSG. This is further buttressed by a negative (-) percentage change relative to MSG across all the groups. The highest percentage change was seen in the extract group (-29.90) followed by the control group (-24.13) while the lowest percentage change was seen in low extract co-treated group (-13.59).
The result as shown in Table 6 showed a significant (p<0.05) decrease in the ALT activity across the co-treated groups with a concomitant negative (-) change in percentage relative to MSG. the highest percentage change was seen in the control group (-40.49) while the lowest percentage change was seen in medium co-treated dose (-14.53).
The result as shown in Table 7 revealed a significant (p<0.05) decrease in the ALP activity across the co-treated groups when compared to the MSG. This is marked with concomitant a negative (-) change in percentage relative to MSG with the highest percentage change seen in the control group (-40.77) followed by extract group (-39.77) while the lowest percentage change was registered by low extract group (-32.82).
The result as shown in Table 8 indicated a significant (p<0.05) dose-dependent increase in the GSH concentration across the co-treated groups when compared to the MSG marked with concomitant a positive (+) change in percentage relative to MSG. the highest percentage change was noticed in the extract group (211.11) while the lowest was noticed in a low co-treated group (46.76).
| Table 1: Mineral content of Tetrapleura tetraptera | |
| Minerals | Bioavailability (mg/100 g–1) |
| Potassium | 5.70± 0.02 |
| Sodium | 663.73±1.27 |
| Calcium | 6.16±0.03 |
| Magnesium | 19.11±0.08 |
|
Values are presented as mean±standard deviation of triplicate determination (n = 3) |
|
| Table 2: Proximate composition of Tetrapleura tetraptera | |
| Proximate parameters | Percentage composition (%) |
| Protein | 19.75±0.05 |
| Ash | 1.65±0.01 |
| Moisture | 6.35±0.01 |
| Fats | 2.42±0.01 |
| Fibre | 1.02±0.01 |
| Carbohydrate | 68.81±0.08 |
Values are presented as mean±standard deviation of triplicate determination (n = 3)
|
|
| Table 3: Vitamin contents of Tetrapleura tetraptera | |
| Vitamins | Bioavailability |
| Vitamin B3 (μg/g) | 21.90±1.21 |
| Vitamin E (mg/100 g–1) | 42.50±2.50 |
| Vitamin B2 (μg/g) | 47.01±1.07 |
| Vitamin B1 (μg/g) | 90.88±4.00 |
| Vitamin A (μg/g) | 315.81±5.83 |
Values are presented as mean±standard deviation of triplicate determination (n = 3)
|
|
Table 4: Phytochemical composition of Tetrapleura tetraptera |
|
| Phytochemicals | Bioavailability (mg/100 g–1) |
| Glycosides | 2.41±0.12 |
| Steroids | 6.86±0.47 |
| Tannins | 10.38±0.62 |
| Terpenoids | 118.73±2.51 |
| Reducing sugar | 416.02±3.74 |
| Alkaloids | 567.17±9.37 |
| Carbohydrates | 595.07±8.53 |
| Flavonoids | 672.66±5.07 |
| Phenolics | 823.07±10.62 |
Values are presented as mean±standard deviation of triplicate determination (n = 3) |
|
Table 5: Effects of TTLEE on AST activity of normal and MSG intoxicated rats |
|||
| GROUPS | AST IU/L | % Change relative to extract | % Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 68.82±0.24 | 23.27 | -13.59 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 68.63±0.36 | 22.92 | -13.83 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 67.40±0.21 | 20.72 | -15.37 |
| Control (feed+water) | 60.42±0.32 | 0.22 | -24.13 |
| TTLEE (200 mg kg–1 b.wt. extract) | 55.83±0.16 | 0.00 | -29.90 |
| MSG (8000 mg kg–1 b.wt. MSG) | 79.64±0.43 | 42.65 | 0.00 |
Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
|||
Table 6: Effects of TTLEE on ALT activity of normal and MSG intoxicated rats |
|||
| GROUPS | ALT IU/L | Change relative to extract | Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 38.23±0.10 | -3.53 | -33.88 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 49.42±0.13 | 27.70 | -14.53 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 41.23±0.22 | 4.04 | -28.69 |
| Control (feed+water) | 34.41±0.18 | -13.17 | -40.49 |
| TTLEE (200 mg kg–1 b.wt. extract) | 39.63±0.21 | 0.00 | -31.46 |
| MSG (8000 mg kg–1 b.wt. MSG) | 57.82±0.43 | 45.90 | 0.00 |
|
Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
|||
| Table 7: Effects of TTLEE on ALP activity of normal and MSG intoxicated rats | |||
| GROUPS | ALP IU/L | % Change relative to extract | % Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 15.21±0.02 | 11.51 | -32.82 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 14.22±0.03 | 4.25 | -37.01 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 15.04±0.01 | 10.34 | -33.57 |
| Control (feed+water) | 13.41±0.01 | -1.69 | -40.77 |
| TTLEE (200 mg kg–1 b.wt. extract) | 13.64±0.03 | 0.00 | -39.75 |
| MSG (8000 mg kg–1 b.wt. MSG) | 22.64±0.13 | 65.96 | 0.00 |
Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
|||
| Table 8: Effects of TTLEE on GSH concentration of normal and MSG intoxicated rats | |||
| GROUPS | GSH mg/dl | % Change relative to extract | % Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 3.17±0.02 | -52.89 | 46.76 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 4.44±0.02 | -33.93 | 85.19 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 4.64±0.01 | -30.95 | 114.80 |
| Control (feed+water) | 4.62±0.01 | -31.25 | 113.89 |
| TTLEE (200 mg kg–1 b.wt. extract) | 6.72±0.01 | 0.00 | 211.11 |
| MSG (8000 mg kg–1 b.wt. MSG) | 2.16±0.01 | -67.98 | 0.00 |
Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
|||
The result as shown in Table 9 significant (p<0.05) dose-dependent increase in the SOD activity across the co-treated groups when compared to the MSG marked with concomitant a positive (+) change in percentage relative to MSG. The highest change was noticed in medium and high co-treated groups (1.50) while the lowest percentage change was noticed in the low co-treated group (0.61).
The result as shown in Table 10 revealed significant (p<0.05) decrease in the CAT activity across the co-treated groups when compared to the MSG marked with concomitant a negative (-) change in percentage relative to MSG for the control group. The highest percentage change was seen in the extract group (-39.75) while the control group had the lowest percentage change (10.55).
The result as shown in Table 11 revealed a marked decrease in the MDA concentration across the co-treated groups when compared to the MSG. This is followed by a concomitant negative (-) change in percentage relative to MSG which is topped by the control group and extract group at (-18.75) while the lowest percentage change was observed in medium extract group (-3.13).
Result as shown in Table 12 showed significantly (p<0.05) higher ALT:AST and a lower AST:ALT ratio across all the groups. The highest ALT:AST ratio was observed in the low extract group while the lowest was observed in the MSG group. This feat is further buttressed in the change relative to MSG and control.
The result as shown in Table 13 indicated significant (p<0.05) higher ALP:AST ratio with lower AST:ALP ratio across all the groups. The highest ALP:AST ratio was observed in the MSG group while the lowest was observed in the medium extract group.
The result as shown in Table 14 indicated significant (p<0.05) higher ALP:ALT ratio with concomitant significant (p<0.05) lower ALT:ALP ratio. The highest ALP:ALT ratio was seen in the low extract group while the lowest ratio was observed in the medium extract group.
| Table 9: Effects of TTLEE on SOD activity of normal and MSG intoxicated rats | |||
| GROUPS | SOD IU/L | % Change relative to extract | % Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 11.38±0.01 | -0.79 | 0.61 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 11.48±0.01 | 0.09 | 1.50 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 11.48±0.01 | 0.09 | 1.50 |
| Control (feed+water) | 11.45±0.01 | -0.17 | 1.24 |
| TTLEE (200 mg kg–1 b.wt. extract) | 11.47±0.01 | 0.00 | 1.42 |
| MSG (8000 mg kg–1 b.wt. MSG) | 11.31±0.01 | -1.40 | 0.00 | Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
| Table 10: Effects of TTLEE on CAT activity of normal and MSG intoxicated rats | |||
| GROUPS | CAT IU/L | % Change relative to extract | % Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 16.66±0.10 | -15.90 | 49.33 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 27.87±0.04 | 40.69 | -15.24 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 29.10±0.21 | 46.90 | -11.50 |
| Control (feed+water) | 29.41±0.12 | 48.46 | -10.55 |
| TTLEE (200 mg kg–1 b.wt. extract) | 19.81±0.11 | 0.00 | -39.75 |
| MSG (8000 mg kg–1 b.wt. MSG) | 32.88±0.32 | 65.98 | 0.00 | Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
| Table 11: Effects of TTLEE on MDA concentration of normal and MSG intoxicated rats | |||
| GROUPS | MDA mg/dl | Change relative to extract | Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 0.30±0.01 | 15.39 | -6.25 |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 0.31±0.01 | 19.23 | -3.13 |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 0.29±0.01 | 11.54 | -9.38 |
| Control (feed+water) | 0.26±0.01 | 0.00 | -18.75 |
| TTLEE (200 mg kg–1 b.wt. extract) | 0.26±0.01 | 0.00 | -18.75 |
| MSG (8000 mg kg–1 b.wt. MSG) | 0.32±0.01 | 23.08 | 0.00 | Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
| Table 12: Effects of TTLEE on AST:ALT of normal and MSG intoxicated rats | |||
| GROUPS | AST:ALT (ALT:AST) | Change relative to extract | Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 1.80 (0.56) | 37.66 (-21.12) | 31.39 (-23.29) |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 1.39 (0.72) | -1.42 (1.41) | 1.46 (-1.37) |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 1.64 (0.61) | 16.31 (-14.09) | 19.71 (-16.44) |
| Control (feed+water) | 1.76 (0.57) | 24.82 (-19.72) | 28.47 (-21.92) |
| TTLEE (200 mg kg–1 b.wt. extract) | 1.41 (0.71) | 0.00 (0.00) | 2.92 (-2.74) |
| MSG (8000 mg kg–1 b.wt. MSG) | 1.37 (0.73) | -2.84 (2.82) | 0.00 (0.00) | Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
| Table 13: Effects of TTLEE on AST:ALP of normal and MSG intoxicated rats | |||
| GROUPS | AST:ALP (ALP:AST) | Change relative to extract | Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 4.53 (0.22) | 9.16 (-8.33) | 28.69 (-21.43) |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 4.83 (0.21) | 16.83 (-12.50) | 37.22 (-25.00) |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 4.48 (0.22) | 7.95 (-8.33) | 27.27 (-21.43) |
| Control (feed+water) | 2.57 (0.22) | -38.07 (-8.33) | 26.42 (-21.43) |
| TTLEE (200 mg kg–1 b.wt. extract) | 4.15 (0.24) | 0.00 (0.00) | 17.90 (-14.29) |
| MSG (8000 mg kg–1 b.wt. MSG) | 3.52 (0.28) | -15.18 (16.67) | 0.00 (0.00) | Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
| Table 14: Effects of TTLEE on ALT:ALP of normal and MSG intoxicated rats | |||
| GROUPS | ALT:ALP (ALP:ALT) | Change relative to extract | Change relative to MSG |
| Low extract (MSG 8000+200 mg kg–1 b.wt. extract) | 2.51 (0.40) | -14.63 (17.65) | -1.57 (2.56) |
| Medium extract (MSG 8000+400 mg kg–1 b.wt. extract) | 3.48 (0.29) | 18.37 (14.71) | 36.47 (-25.64) |
| High extract (MSG 8000+600 mg kg–1 b.wt. extract) | 2.74 (0.37) | -6.80 (8.82) | 7.45 (-5.13) |
| Control (feed+water) | 2.57 (0.39) | -12.59 (14.71) | 0.78 (0.00) |
| TTLEE (200 mg kg–1 b.wt. extract) | 2.94 (0.34) | 0.00 (0.00) | 15.29 (-12.82) |
| MSG (8000 mg kg–1 b.wt. MSG) | 2.55 (0.39) | -13.27 (14.71) | 0.00 (0.00) | Values are Mean±SEM for n = 4. Difference considered statistically significant at p<0.05.+Denotes higher by, -Denotes lower by MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extract |
The Photomicrograph of the liver section of group A rats (Fig. 3) revealed a normal flow of blood with partial congestion of the central vein while the photomicrograph of the liver section of group B rats (Fig. 4) showed a normal flow of blood with mild congestion of the central vein.
The photomicrograph of the liver section of group C rats (Fig. 5) indicated a normal flow of blood with mild congestion of the central vein and the photomicrograph of the liver section of group D (Fig. 6) rats showed well-preserved architecture with no histopathological lesion and normal central vein. The photomicrograph of the liver section of group E (Fig. 7) rats suggested a normal flow of blood with no congestion of the central vein and mild steatosis. However, the photomicrograph of the liver section of group F rats (Fig. 8) indicated full congestion of the central vein.
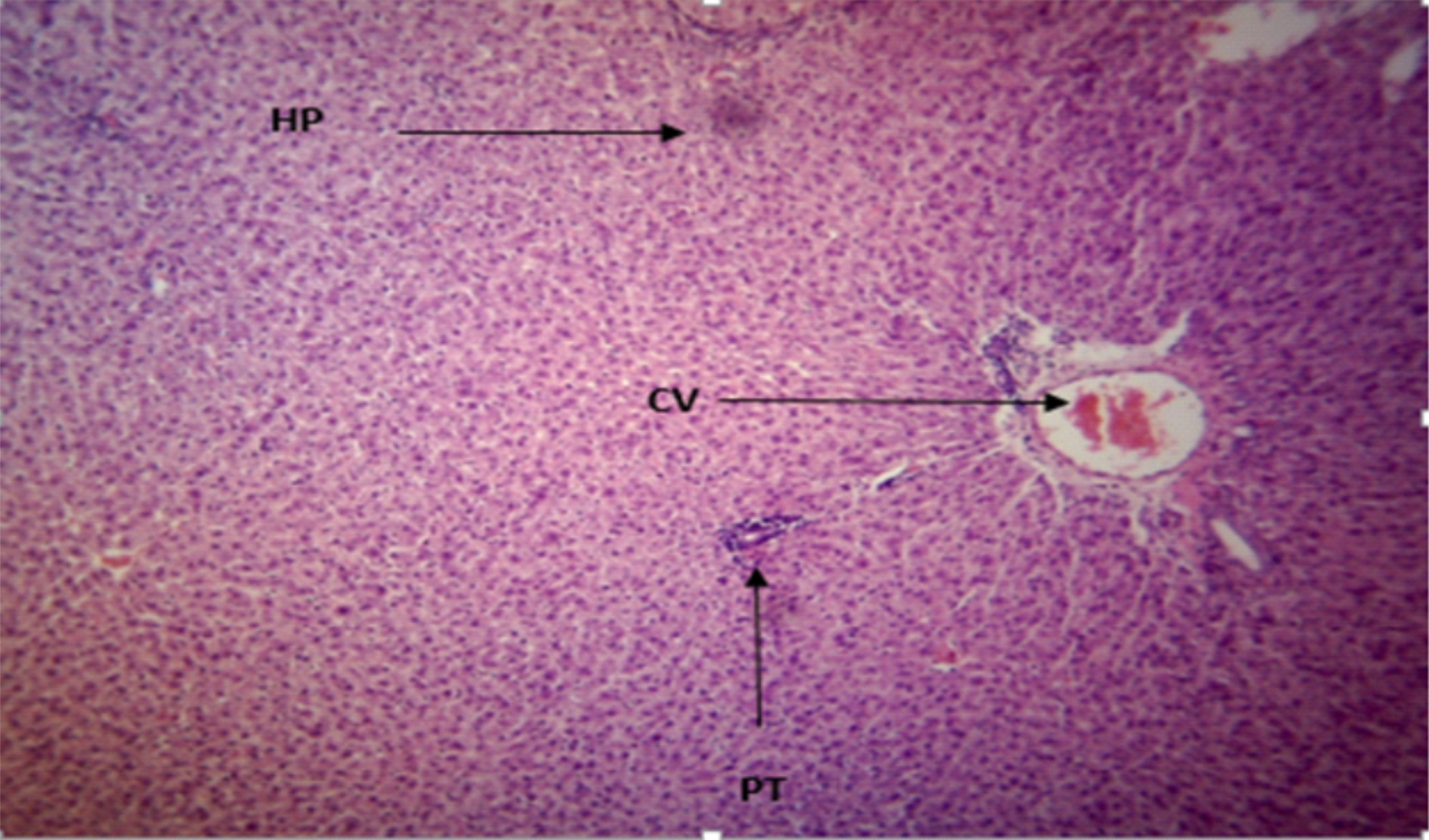 |
Fig. 3: T he Photomicrograph of the liver section of group A (low extract group) rats Plates of a photomicrograph of rat liver section. (Hematoxylin and Eosin) stained ×400 Keys: PT = Portal Triad, HP= Hepatocyte, N= Necrosis, F= Fibrosis, CV= Central Vein MSG = monosodium Glutamate TTLEE = Tetrapleura tetraptera leaves ethanol extrac |
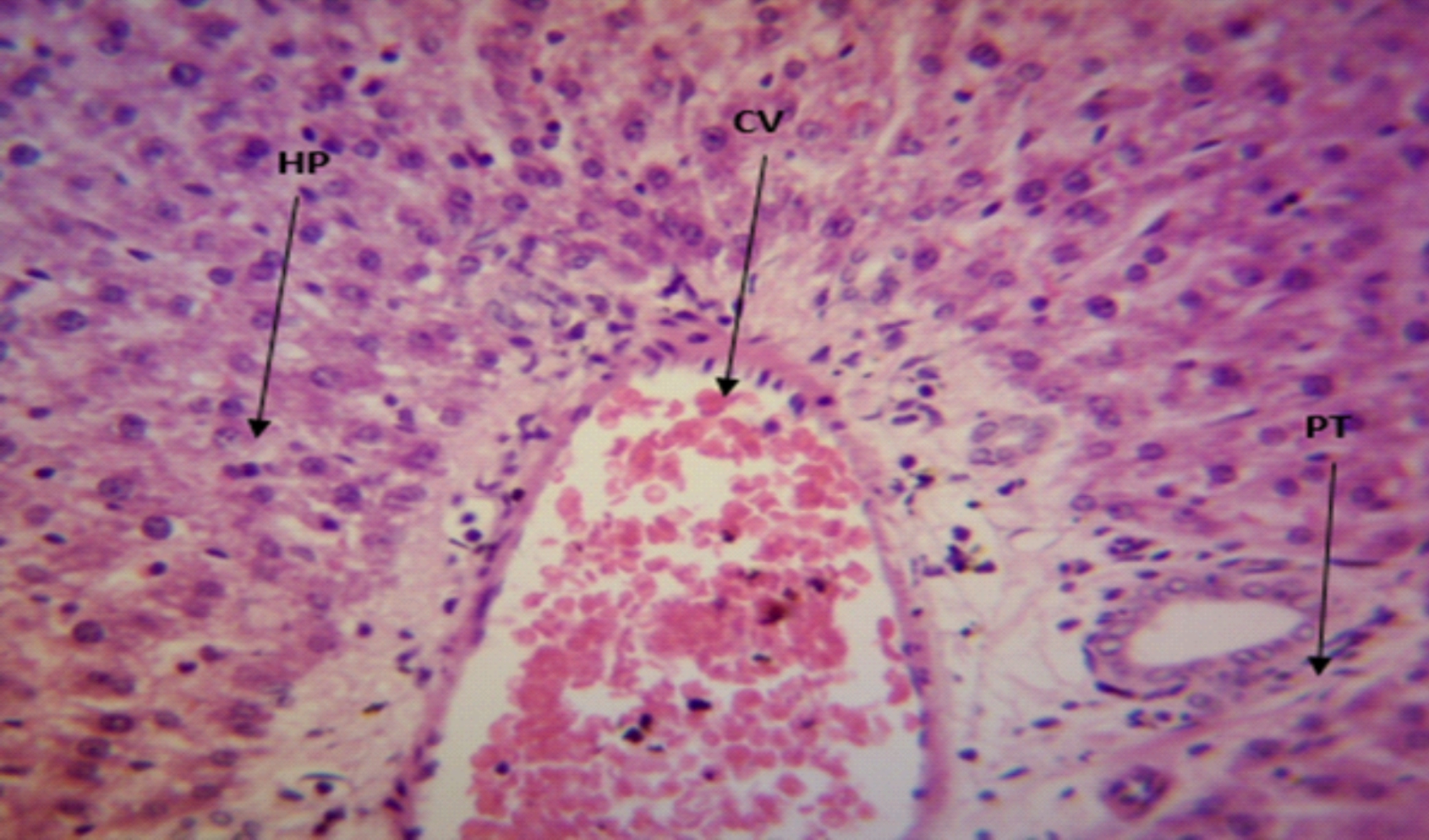 |
Fig. 4: The Photomicrograph of the liver section of group B (medium extract group) rats Keys: PT = Portal Triad, HP= Hepatocyte, N= Necrosis, F= Fibrosis, CV= Central Vein |
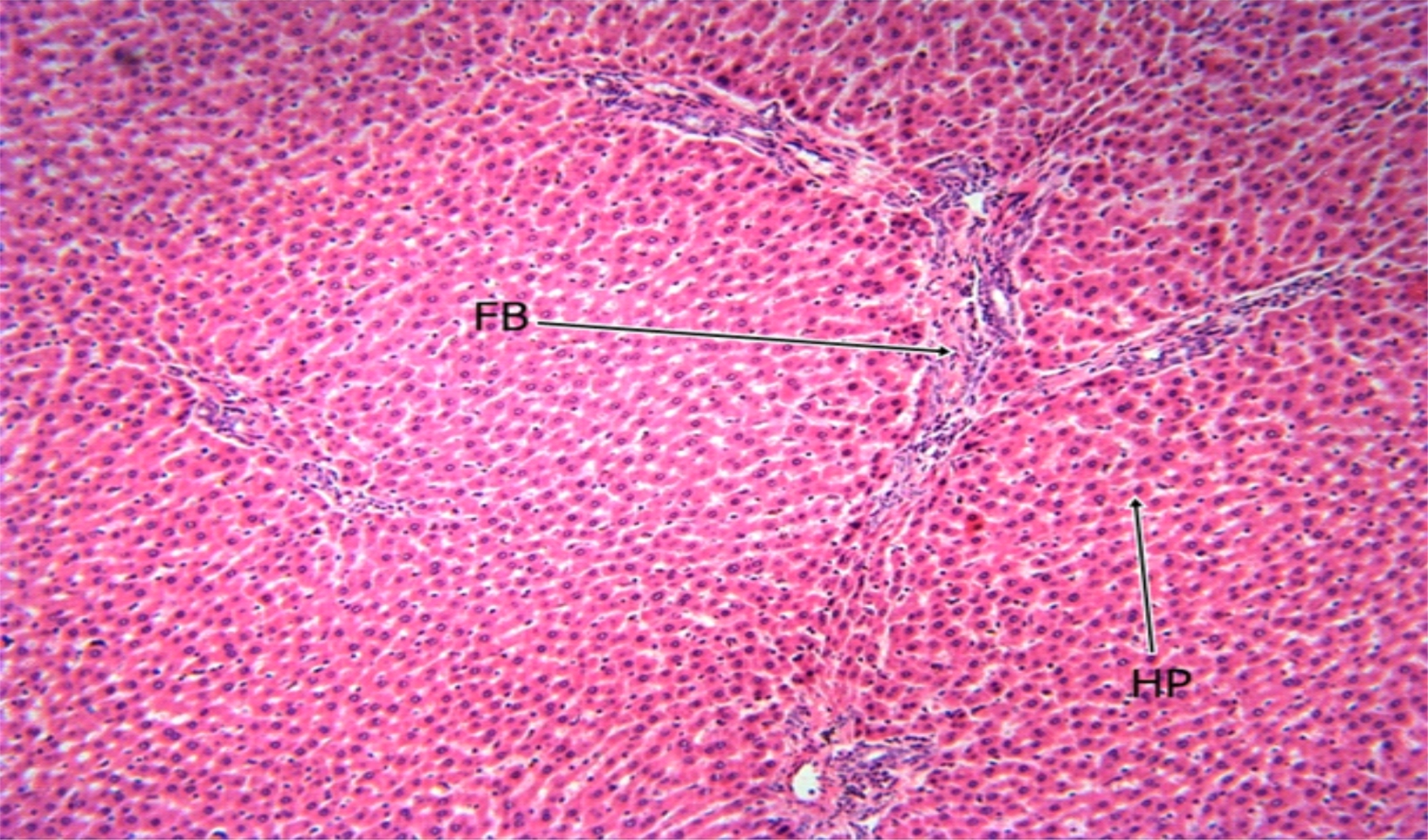 |
Fig. 5: The Photomicrograph of the liver section of group C (high dose extract group) rats Keys: PT = Portal Triad, HP= Hepatocyte, N= Necrosis, FB= Fibrosis, CV= Central Vein |
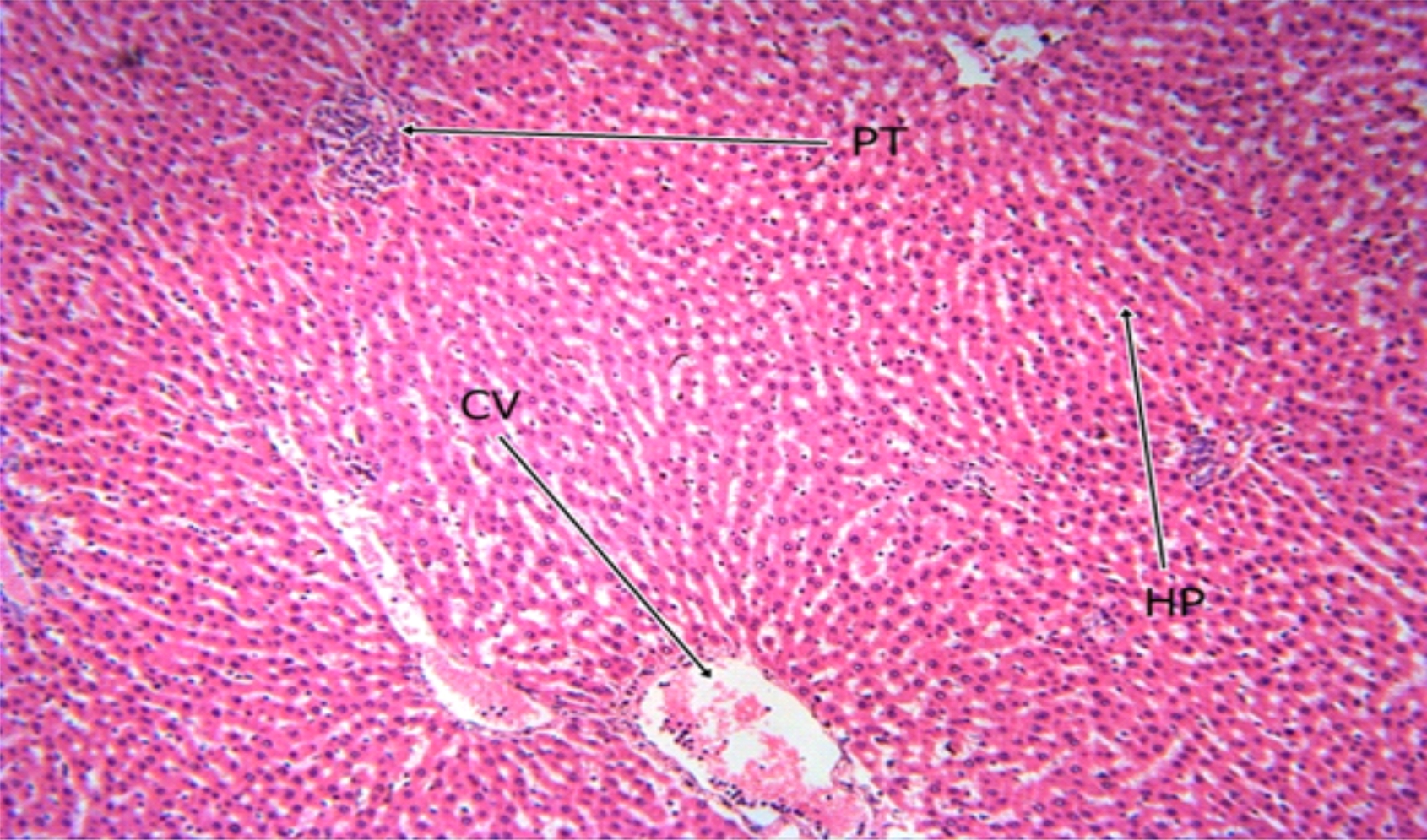 |
Fig. 6: The Photomicrograph of the liver section of group D (control) rats Keys: PT = Portal Triad, HP= Hepatocyte, N= Necrosis, F= Fibrosis, CV= Central Vein |
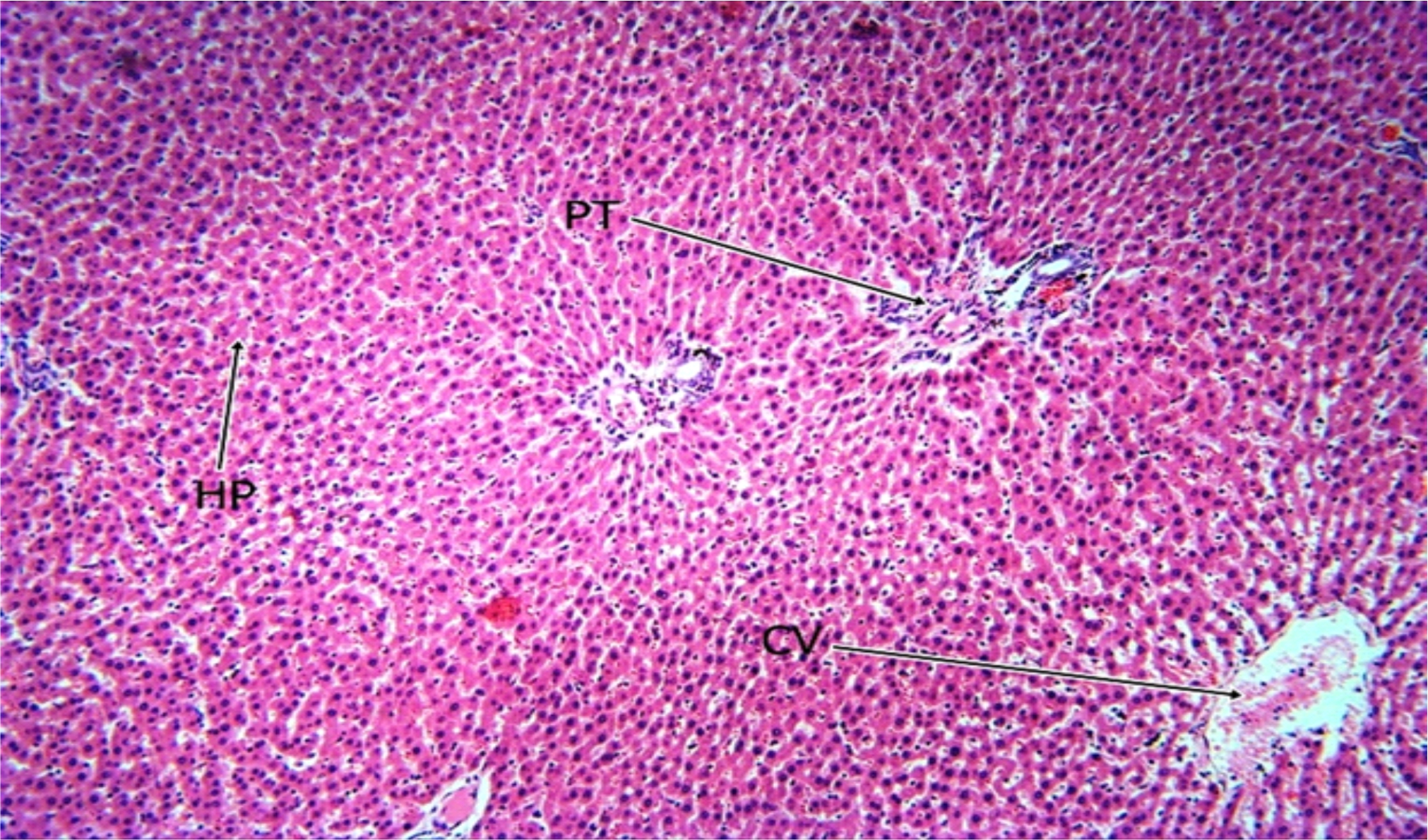 |
Fig. 7: The Photomicrograph of the liver section of group E (extract group) rats Keys: PT = Portal Triad, HP= Hepatocyte, N= Necrosis, F= Fibrosis, CV= Central Vein |
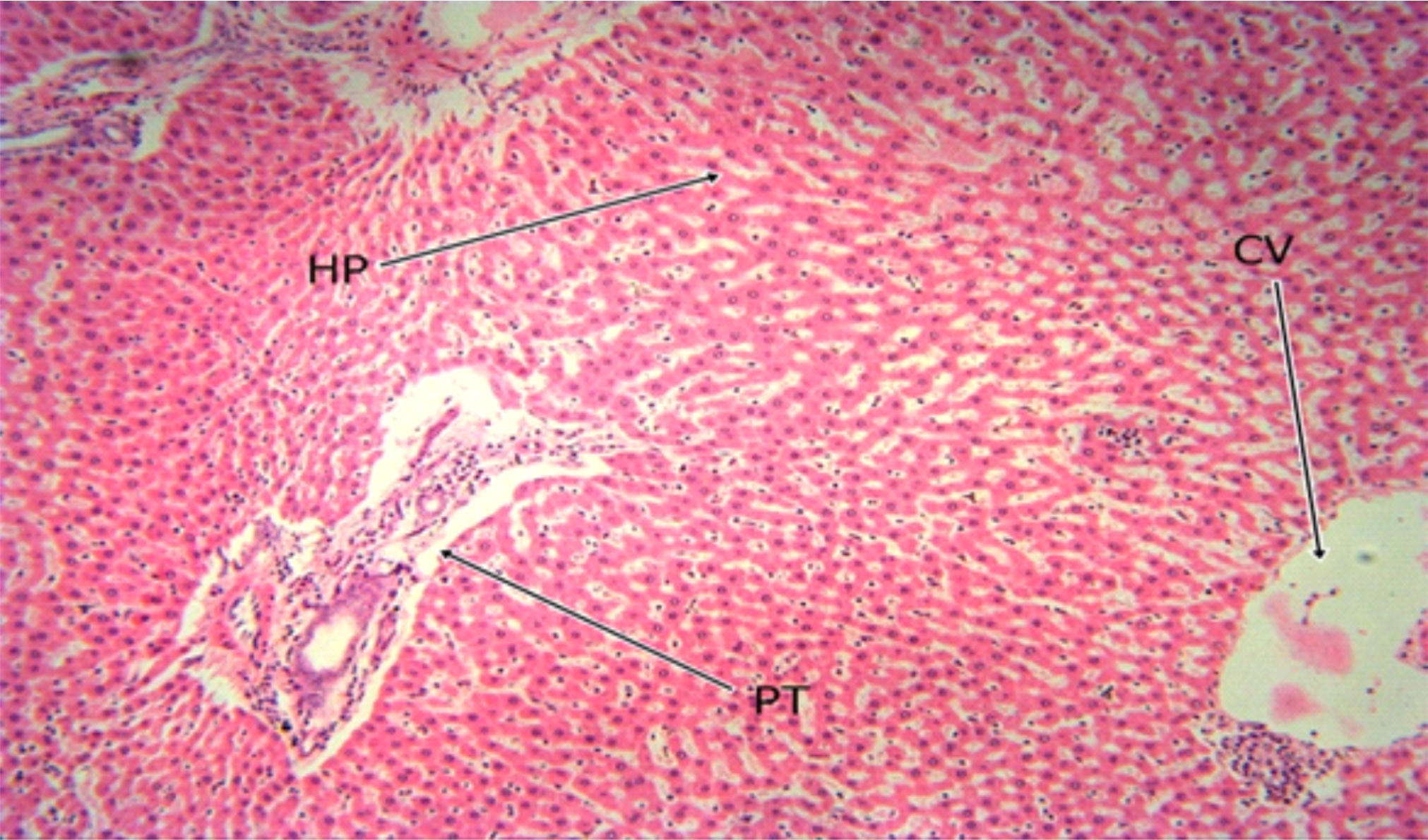 |
Fig. 8: The Photomicrograph of the liver section of group F (MSG group) rats. Keys: PT = Portal Triad, HP= Hepatocyte, N= Necrosis, F= Fibrosis, CV= Central Vein |
DISCUSSION
Monosodium glutamate consumption is not restricted and stands a high chance of inadvertent abuse28 warranting this study aimed at investigating the Nutraceutical composition of Tetrapleura Tetraptera Leaves and its effect on hepatic histo-morphology, serum bio-functional parameters and serum antioxioxidant activities in Monosodium Glutamate-intoxicated Rats.
High presenceof flavonoids, tannins, alkaloids, and other chemical substances in Tetrapleura tetraptera suggest high medicinal potentials and could be the reasons for its antioxidant properties. Flavonoids are found in most plants and have been found to have many antioxidants, anti-allergic, anti-inflammatory and antiviral properties29,30 while alkaloids have a wide range of pharmacological activities including anti- malarial, anti-bacterial and anti-hyperglycemic activities31,32. The flavonoid concentration of Tetrapleura tetraptera was higher than that reported by33 and the alkaloid concentration of Tetrapleura tetraptera was higher than that reported by34. This could suggest that the leaves has high antioxidant potential hence, agrees with the findings of35.
Minerals play important roles in maintaining homeostasis in the living system and in the maintenance of muscle tone and body electrolytes36. The sodium composition of Tetrapleura tetraptera leaves was higher than that reported by37 while the magnesium composition was higher than that reported by38. The difference in these values may be attributed to variation in the availability of soil micronutrient to plants in different locations. The high mineral composition of Tetrapleura tetraptera as implicated in the result could suggest possible role in the maintenance of muscle tone and body electrolytes.
As part of the neutraceutical study investigated on the Tetrepleura tetraptera leaves, the phytochemical composition was estimated. Tetrepleura tetraptera leaves have high moisture content which may reduce the durability or storage stability of the sample flour. High moisture content subjects food to increased microbial spoilage deterioration and short shell life39. The ash content (1.65%) indicates that the flour could be a rich source of inorganic matter since the ash content is used to estimate the total amount of minerals present within the food. High carbohydrate content of Tetrepleura tetraptera leaves could suggest its ability in stability of plasma glucose level hence preventing easy degradation of body protein to obtain energy. The presence of lipid in the Tetrepleura tetraptera leaves is suggestive of its nutritional value. Lipids provide an excellent source of energy enhances transport of fat soluble vitamins and protects internal organs.
Vitamins are organic substances required in a minute measure for proper functioning of cells and could play vital roles in metabolic regulations and also modify the activities of enzymes40. Vitamin B2 is a necessary intermediate for carbohydrate metabolism and act as an antioxidant to provide some protection from oxidative stress and free radicals41. The Vitamin B1 content of Tetrepleura tetraptera is higher than that reported by42 suggesting that it could be a good source of vitamin B1.
Oxidative stress has indeed been implicated in the pathogenesis of various disease conditions in humans43. Monosodium glutamate induces toxicity by causing oxidative stress in treated animals with characteristic lipid peroxidation, and possible damage to the liver7. The In-vitro antioxidant study of Tetrepleura tetraptera leaves indicated significant higher scavenging of DPPH free radical ion when compared to ascorbic acid used as standard. The FRAP assay showed higher ability to reduce TPRZ-Fe (III) complex to TPRZ-Fe (II) with values when compared to that obtained for Gallic acid standard.
The alteration of hepatic membrane integrity is usually accompanied by leakage of enzymes into the blood44 and the estimation of these enzymes in tissue and the body fluids play a significant role in disease investigation and diagnosis45 and aid in determination of organ dysfunction long before it is picked up by the conventional histological technique.
The activities of AST; found in the liver and muscles and ALT; a more specific enzyme in the determination of hepatic assault are all significantly (p<0.05) increased in the MSG group indicating hepatic injury and agrees with the result of6. However, concomitant administration of MSG with TTLEE showed reduced activity of the enzymes suggesting its hepetoprotective potential. This could be attributed to its high antioxidant potentials since MSG have been found to induce hepatic challenge through initiation of oxidative stress. This fact is further confirmed by the result of the serum antioxidant assay which showed positive (+) % change in GSH concentration relative to MSG, negative (-) % change in CAT activity relative to MSG and negative (-) % change in MDA concentration relative to MSG suggesting high antioxidant potential of TTLEE (Table 8, 10 and 11). This observed trend extends to the calculated ratios (AST:ALT, AST:ALP, ALT:ALP) and stamped by the hepatic micrograph indicating improved structural architecture of the hepatoctes following co-administration of MSG and TTLEE.
CONCLUSION
It can be concluded that Tetrapleura tetrapterahas high mineral content and vitamin
composition. It has very high phenol and flavonoids which are known antioxidants. It
has relatively high FRAP and DPPH activity and was able to ameliorate the hepatic injury
induced on the animals by MSG.
SIGNIFICANCE STATEMENT
This study discovered the rich phytochemical, vitamin and mineral composition of Tetrapleura tetraptera leaves. This is important since there is increase in the incidence of hepatic dysfunctions and search for natural remedies for diseases. This study will help the researchers to identify the use of Tetrapleura tetraptera as antioxidant supplement and possible natural remedy in the management liver dysfunction. Thus a new theory on of Tetrapleura tetraptera as antioxidant supplement and possible natural remedy in the management liver dysfunction may be arrived at.
REFERENCES
- Yogalakshmi, B., P. Viswanathan and C.V. Anuradha, 2010. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology, 268: 204-212.
- Udourioh, G.A. and M.F. Etokudoh, 2014. Essential oils and fatty acids composition of dry fruits of Tetrapleura tetraptera. J. Appl. Sci. Environ. Manage., 18: 419-424.
- Jimmy, E.O. and A.J.D. Ekpo, 2016. Upgrading of lethal dose of Tetrapleura tetraptera extract enhances blood cell values. J. Hematol. Thrombo. Dis., 04: 256-258.
- Park, C.H., S.H. Choi, Y. Piaoa, S. Kim and Y. Lee et al., 2000. Glutamate and Aspartate impair memory retention and damage hypothalamic neurons in adult mice. Toxicol. Lett., 115: 117-125.
- Gobatto, C.A., M.A. Mello, C.T. Souza and I.A. Ribeiro, 2002. The monosodium glutamate (MSG) obese rat as a model for the study of exercise in obesity. Res. Commun. Mol. Pathol. Pharmacol., 111: 89-101.
- Egbuonu, A.C.C., C.I. Opara, D. Akachukwu and U.B. Onyedikachi, 2018. Effect of ethanolic extract of avocado pear (Persea americana) seed on normal and monosodium glutamate-compromised Rats` hepatic histo-morphology and serum bio-functional parameters. Res. J. Environ. Sci., 12: 53-62.
- Obidike, I.J. and A.C. Egbuonu, 2019. Effects of ethanol extract of cocoa (Theobroma cacao) pod on normal and monosodium glutamate-intoxicated rats' hepatic histo-morphology, serum bio-functional parameters and serum antioxidant activities. Int. J. Recent Res. Applied Stud., 6: 1-13.
- Broberger, C., 2005. Brain regulation of food intake and appetite: molecules and networks. J. Intern. Med., 258: 301-327.
- Dugasani, S., M.R. Pichika, V.D. Nadarajah, M.K. Balijapalli, S. Tandra and J.N. Korlakunta, 2010. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol., 2: 515-520.
- Tanumihardjo, S.A., 2011. Vitamin A: biomarkers of nutrition for development. Am. J. Clin. Nutr., 94: 658S-665S.
- Olson, J.H., J.C. Erie and S.J. Bakri, 2011. Nutritional supplementation and age-related macular degeneration. Semin. Ophthalmol., 26: 131-136.
- AOAC, 1995. Official Method of the Association of Official Analytical Chemists. 16th Edn., AOAC International, Arlington, USA.
- Otitoju, G.T.O., 2009. Effect of dry and wet milling processing techniques on the nutrient composition and organoleptic attributes of fermented yellow maize (Zea mays). Afr. J. Food Sci., 3: 113-116.
- AOAC., 1990. Official Methods of Analysis of the Association of Official Analytical Chemists. 13th Edn., Association of Official Analytical Chemist, Washington, DC., USA.
- Sofowora, A., 1993. Medicinal Plants and Traditional Medicine in Africa. 2nd Edn., Spectrum Books Ltd., Ibadan, Nigeria.
- Harborne, A.J., 1998. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis. 3rd Edn., Springer, Netherlands.
- Bohm, B.A. and M.R. Koupai-Abyazani, 1994. Flavonoids and condensed tannins from leaves of Hawaiian Vaccinium reticulatum and V. calycinum (Ericaceae). Pac. Sci., 48: 458-463.
- Onwuka, G.I., 2005. Food Analysis and Instrumentation: Theory and Practice. 1st Edn., Naphthali Prints, Lagos, Nigeria, pp: 1-219.
- Benzie, I.F.F. and J.J. Strain, 1996. The Ferric Reducing Ability of Plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal. Biochem., 239: 70-76.
- Brand-Williams, W., M.E. Cuvelier and C. Berset, 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol., 28: 25-30.
- Reitman, S. and S. Frankel, 1957. A colormetric method for the determination of serum glutamate oxaloacetate transaminase. Am. J. Clin. Pathol., 28: 56-63.
- Englehardt, V.A. 1970. Measurement of alkaline phosphatase. Aerztl Labour 1970. 16:42.
- Vashney, R. and R.F. Kale, 1990. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol., 58: 733-743.
- Aebi, H.E., 1983. Catalase. In: Methods of Enzymatic Analysis, Bergmeyer, H.U., J. Bergmeyer and M. Grassl (Ed.). 3rd Edn., Verlag Chemie, Weinhem, pp: 273-286.
- Habig, W.H., M.J. Pabst and W.B. Jakoby, 1974. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem., 249: 7130-7139.
- Clayden, E.C., 1967. Practical Section Cutting and Staining. 4th Edn., J and E Church Hill, U.K.
- Thomas, M., K.S. Sujatha and S. George, 2009. Protective effect of Piper longum Linn. on monosodium glutamate induced oxidative stress in rats. Indian J. Exp. Biol., 47: 186-192.
- Egbuonu, A.C.C., 2015. Assessment of some antinutrient properties of the watermelon (Citrullus lanatus) rind and seed. Res. J. Environ. Sci., 9: 225-232.
- Cushnie, T.P.T., B. Cushnie and A.J. Lamb, 2014. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents, 44: 377-386.
- Manner, S., M. Skogman, D. Goeres, P. Vuorela and A. Fallarero, 2013. Systematic exploration of natural and synthetic flavonoids for the inhibition of Staphylococcus aureus biofilms. Int. J. Mol. Sci., 14: 19434-19451.
- Kittakoop, P., C. Mahidol and S. Ruchirawat, 2014. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis and smoking cessation. Curr. Top. Med. Chem., 14: 239-252.
- Qui, S., H. Sun, A.H. Zhang, H.H. Xu, G.L. Yan, Y. Han and X.J. Wang, 2014. Natural alkaloids: basic aspects, biological roles, and future perspectives. Chinese J. Nat. Med., 12: 401-406.
- Uyoh E.A., E.E. Ita and G.E. Nwofia, 2013. Evaluation of chemical composition of Tetrapleura tetraptera (Schum and Thonn.) Taub. From Cross River State, Nigeria. Int. J. Med. Aromatic Plants, 3: 386-394.
- Abii, T.A. and A. Elegalam, 2007. Investigation into the chemical composition of the dry fruit of Tetrapleura tetraptera (Ubukirihu). J. Food Technol., 3: 229-232.
- Akin-Idowu R.A., D.O. Ibitoye, T.A. Olufemi and O.T. Adeniyi, 2011. Chemical composition of the dry fruit of Tetrapleura tetraptera and its potential impact on human health. J. Herbs, Spices Med. Plants, 17: 52-61.
- Olusanya, J.O., 2008. Proteins. In: Essentials of Food and Nutrition, Olusanya, J.O., Apex Books Limited, Lagos, pp:13-21.
- Akintola, O.O., 2015. Nutritional and medicinal importance of Tetrapleura tetraptera fruits (ARIDAN). Afr. J. Sci. Res., 6: 33-38.
- Edeoga, H.O., G. Omosun and L.G. Uche, 2006. Chemical composition of Hyptis suaveolens and Ocimum gratissimum hybrids from Nigeria. Niger. Afr. J. Biotechnol.
- Adepoju, O.T. and L.O. Onasanya, 2008. Nutrient composition and antinutritional factors of Dialium guineense willd fruit pulp. Ife. J. Sci. 10: 33-37.
- Chikezie, P.C., E.N. Agomuo, and B.A. Amadi, 2008. Proximate analysis. In: Biochemistry Practical/Research Method, Chikezie, P.C., E.N. Agomuo, and B.A. Amadi, Megasoft Publishers, Owerri, pp: 8-21.
- Powers, H.J., 2003. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr., 77: 1352-1360.
- Aderonke, A.M., J.B. Fashakin and S.I. Ibironke, 2014. Determination of mineral contents, proximate composition and functional properties of complementary diets prepared from maize, soybean and pigeon pea. Am. J. Nutr. Food Sci., 1: 53-56.
- Hybertson, B.M., B. Gao, S.K. Bose and J.M. McCord, 2011. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med., 32: 234-246.
- El Hilaly, J., Z.H. Israili and B. Lyoussi, 2004. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol., 91: 43-50.
- Yakubu, M.T., A.A. Adesokan and M.A. Akanji, 2006. Biochemical changes in the liver, kidney and serum of rat following chronic administration of cimetidine. Afr. J. Biomed. Res., 9: 213-218.
How to Cite this paper?
APA-7 Style
E.I,
C., I.J,
O. (2020). Nutraceutical, Antioxidant and Hepatic Histomorphological Effects of Tetrapleura tetraptera Leaves in Monosodium Glutamate-intoxicated Rats. Asian Journal of Emerging Research, 2(4), 223-238. https://doi.org/10.3923/AJERPK.2020.223.238
ACS Style
E.I,
C.; I.J,
O. Nutraceutical, Antioxidant and Hepatic Histomorphological Effects of Tetrapleura tetraptera Leaves in Monosodium Glutamate-intoxicated Rats. Asian J. Emerg. Res 2020, 2, 223-238. https://doi.org/10.3923/AJERPK.2020.223.238
AMA Style
E.I
C, I.J
O. Nutraceutical, Antioxidant and Hepatic Histomorphological Effects of Tetrapleura tetraptera Leaves in Monosodium Glutamate-intoxicated Rats. Asian Journal of Emerging Research. 2020; 2(4): 223-238. https://doi.org/10.3923/AJERPK.2020.223.238
Chicago/Turabian Style
E.I, Chita, and Obidike I.J.
2020. "Nutraceutical, Antioxidant and Hepatic Histomorphological Effects of Tetrapleura tetraptera Leaves in Monosodium Glutamate-intoxicated Rats" Asian Journal of Emerging Research 2, no. 4: 223-238. https://doi.org/10.3923/AJERPK.2020.223.238

This work is licensed under a Creative Commons Attribution 4.0 International License.



