Bisphenol-A Exposure Causes Increased Oxidation of LDL and Its Abduct in Rats
| Received 18 Feb, 2021 |
Accepted 03 Apr, 2021 |
Published 15 Jun, 2021 |
ABSTRACT
Background and Objectives: Living organisms are exposed to oxidant agents constantly from both endogenous and exogenous sources. One such oxidant agent is Bisphenol-A (BPA) and its exposure is capable to modify biomolecules and induce damages. Bisphenol-A (BPA) is a contaminant with increasing exposure. It exerts toxic effects on cells. This study investigates the possibility of BPA exposure on Low-Density Lipoprotein (LDL) perturbations at prevailing low exposure doses in female albino Wistar rats, following exposure for three (3) month. Materials and Methods: Total 12 groups were formed; out of which 11 experimental groups, each containing 10 non-pregnant female rats were administered; 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1 mg of BPA kg-1 b.wt.day-1. The 12th control group was given water. Blood was collected from animals at the end of every week of the study and serum sample specimens analyzed by routine diagnostic procedures for oxidized LDL such as Malondialdehyde modified-LDL (MDA-LDL), Oxidized Phospholipids-LDL (OX-PL LDL), N (epsilon) (carboxymethyl) lysine-modified-LDL (CML LDL) and 4-hydroxynonenal-LDL (HNE-LDL) using autochemical analyzer. Results: Significantly increased concentrations of serum oxidized LDL such as MDA-LDL, OX-PL LDL, CML LDL and HNE-LDL were observed at all concentrations of BPA exposure. Conclusion: Bisphenol-A alters oxidized LDL such as MDA-LDL, OX-PL LDL, CML LDL and HNE-LDL balance and causes disturbance of internal oxidative statues.
INTRODUCTION
Bisphenol-A (BPA) is a known endocrine disruptor that is ubiquity in our environment. BPA enters the body by the ingestion of contaminated food and beverages. It leaks from polycarbonate plastics and penetrates the body through the skin1,2 or inhalation3,4. Because BPA is well absorbed into the body by ingestion, pregnant women, infants and young children are particularly vulnerable to BPA. The risk of adverse health effects may be due to the increased absorption and decreased excretion of BPA from the body5, and also due to several factors, such as e.g., body weight, metabolic rate. BPA provoked an increase in body weight6, and adipose tissue weight7, alteration in adipogenesis and an increase in white adipose tissue and overexpression of some adipogenic genes8 and increase lipid accumulation in the differentiating adipocytes and up-regulates the expression of adipocyte proteins through the activation of glucocorticoid receptor9. BPA suppresses low glucose-induced intracellular calcium oscillation on α-cells ex vivo10, increase the activation of the transcription factor cAMP (cyclic adenosine monophosphate) response element-binding protein (CREB)3, abnormal levels of the liver enzyme γ-glutamyl-transferases, alkaline phosphatase and lactate dehydrogenase11,12.
BPA has been reported to have adverse health effects on the developing reproductive organs, fetal BPA exposure has been shown to decrease the efficiency of sperm production in male mice offspring8. Recent data of Park et al.13 showed that BPA increased human ovarian cancer cell proliferation. BPA induces toxic effect on non-reproductive vital organs and absorption of BPA causes extensive damage to the liver and kidney11,14, the formation of multinucleated giant cells in liver hepatocytes, DNA adduct and induce the production of free radicals in rat hepatocytes15. This study aims to unveil the possible effects of Bisphenol-A on oxidation of lipid in female Wistar albino rats.
MATERIALS AND METHODS
Study area: The study was carried out at Applied Biochemistry Lab, NnamdiAzikiwe University, Awka, Nigeria and Biochemistry Lab, Gregory University Uturu, Abia State, Nigeria from November 2017-March, 2018.
Methodology: Total 110 non-pregnant female rats of age 5 weeks were acclimatized in the laboratory for 7 days and randomly divided into 11 experimental groups of 10 rats each and respectively administered; 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1 mg of BPA kg-1 b.wt.day-1. The first group which served as control did not receive any treatment but distilled water instead. The graded doses of BPA were dissolved in distilled water and administered by oral gavage using intubation cannula (Lars Medicare Pvt. Ltd, New Delhi, India). Blood was obtained from the tail of the various groups by capillary action weekly, after BPA administration for 13 weeks. Blood samples were processed for clinical assay.
Animals have housed in aluminium wire-mesh cages in a well-ventilated animal house with a 12 hrs dark/light cycle and at room temperature and were provided commercial rat pellets (Vital feed from Vital group of Company, Nigeria) and water ad libitum.
At the end of the experiments serum CML-LDL, MDA-LDL, HNE-LDL and OXPL-LDL were assayed using an Autochemicalanalyser (DxC 800 and LX20 auto-analyzer chemistry analyser Beckman coulter, USA). All reagents were commercially obtained as already prepared kits. The kits for CML-LDL, MDA-LDL, HNE-LDL and OXPL-LDL were purchased from OXI- select (cell Biolabs, inc., USA). Individual tests were carried out according to the kit specifications.
Statistical analysis: Differences between obtained values (mean±SD) were carried out by one-way analysis of variance (ANOVA) using SPSS software version 20 followed by the Tukey-Kramer multiple comparison test. At p≤0.05 was taken as a criterion for a statistically significant difference.
RESULTS
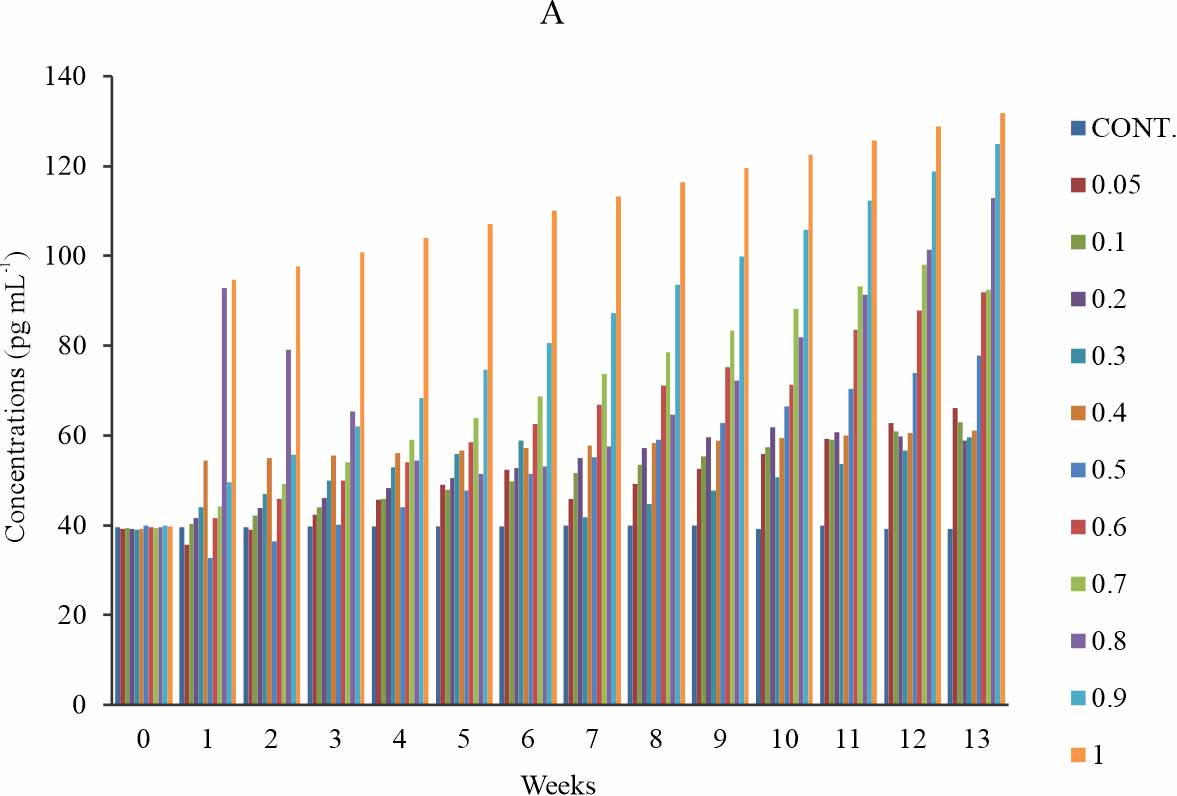
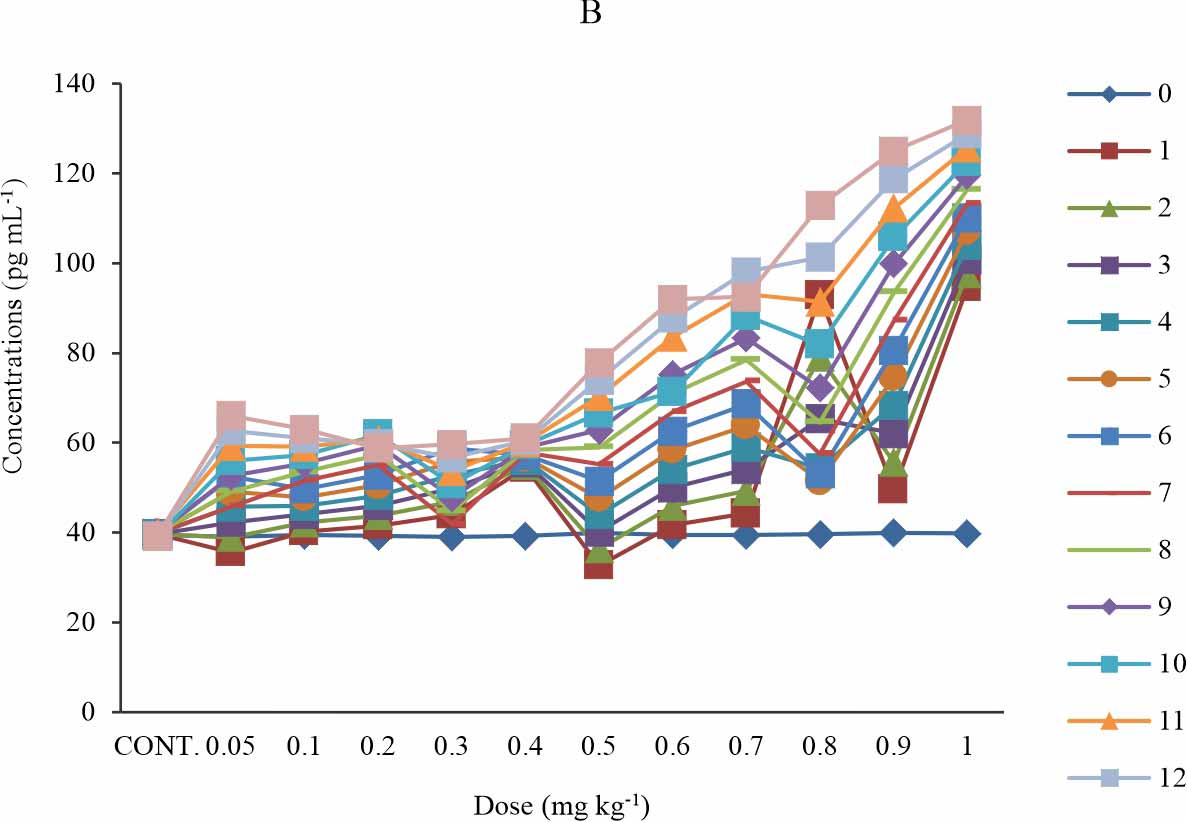
Oxidised phospholipid (OXPL-LDL): There is a significant increase in the OXPL-LDL level when compared with the control p≤0.05 (Fig. 1a). The significance was observed at all weeks in all test groups except for group 6 (0.5 mg kg-1) at week 1-5; then group 1-4 (0.05-0.3 mg kg-1) shows a significant increase only at week 9-13 (Fig. 1b). In the first four (1-4) weeks of exposure to BPA, the groups that were administered 0.05-0.4 mg kg-1 of BPA showed a dose-dependent increase of the oxidised phospholipid level (Fig. 1a). The group that was exposed to 0.9 mg kg-1 of BPA showed lower oxidised phospholipid level relative to that 1 mg kg-1 at all time of exposure and also, The group that was exposed to 0.6 mg kg-1 of BPA showed lower oxidised phospholipid level relative to that 0.7 mg kg-1 at all time of exposure (Fig. 1a). The group that received 1mg kg-1 showed the highest level of oxidised phospholipid at all time of exposure (Fig. 1a-b). At the week 1st and 2nd week of exposure, the serum level of oxidised phospholipid declined below that of control and week 0 for the groups exposed to 0.05, 0.1 and 0.5 mg kg-1 of BPA. (Fig. 1b). The time-sensitive effect observed in Fig. 1b was mild at 0.4 mg kg-1 and a relatively time-dependent effect was observed (Fig. 1b).
 |
||||||
| |
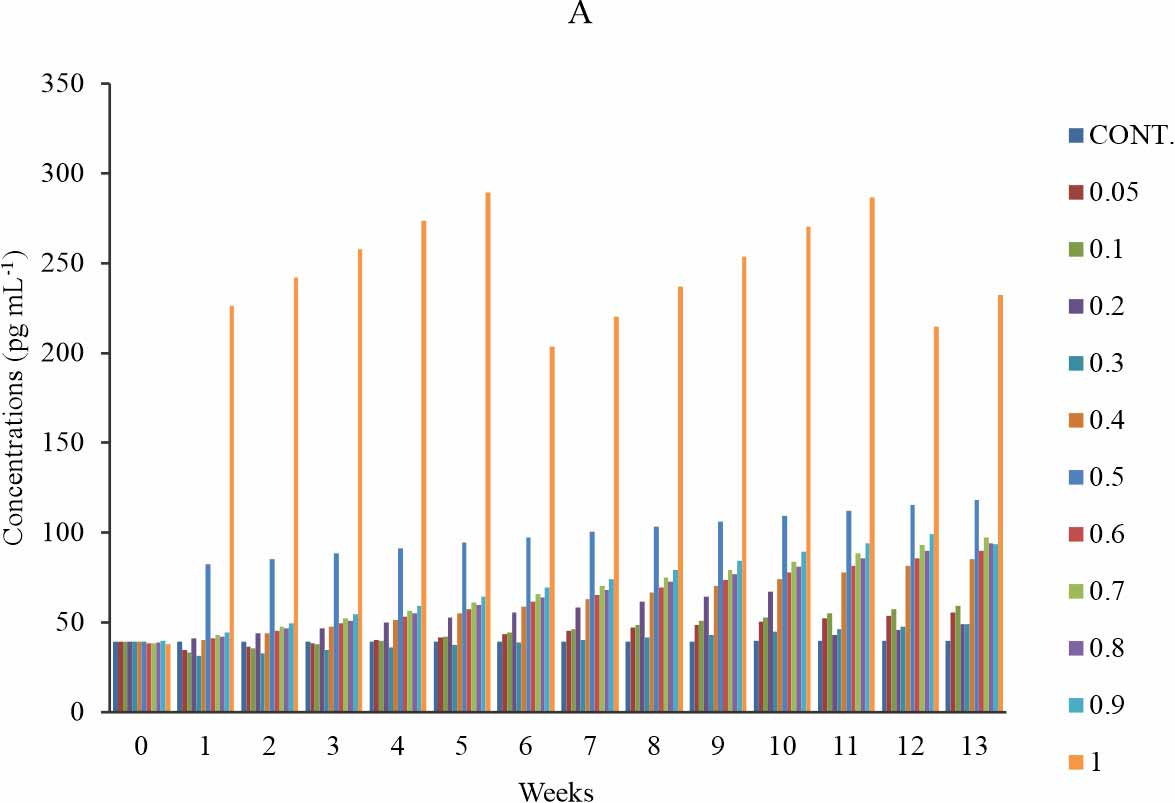
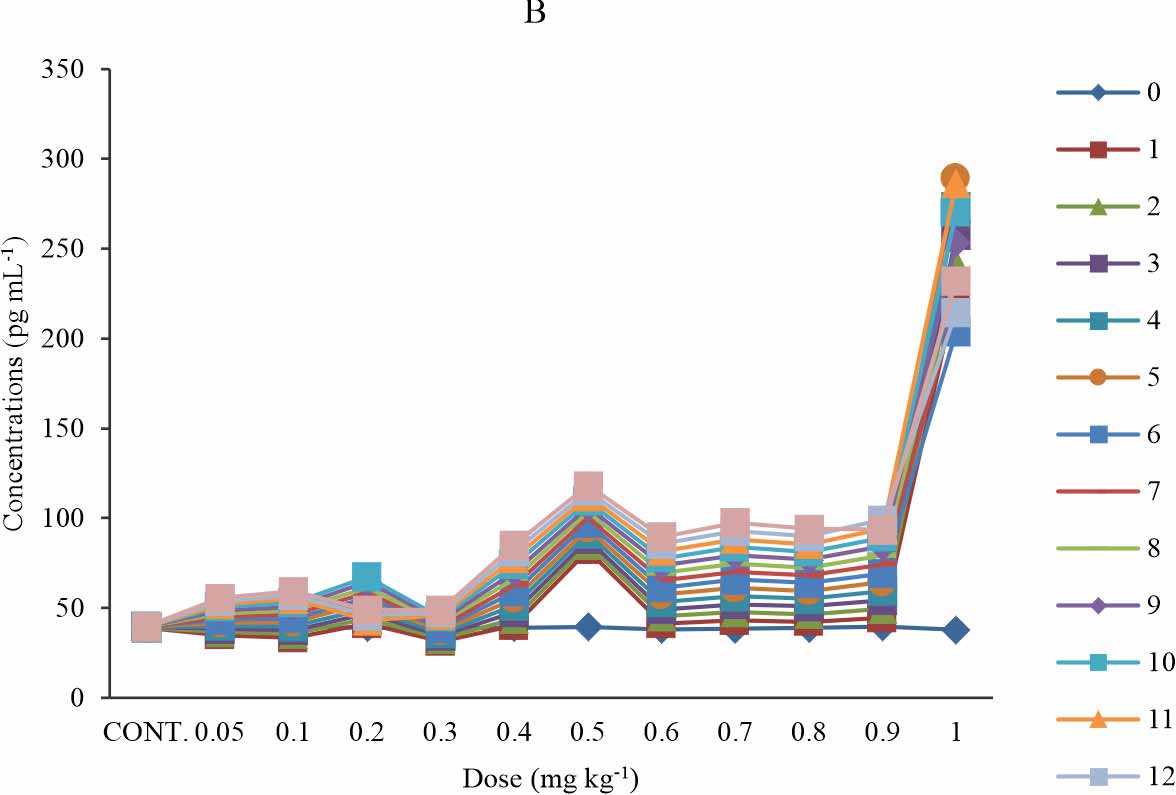
MDA-LDL: There is a significant increase in the MDA-LDL level when compared with the control at p≤0.05 (Fig. 2a), in groups 6 (0.5 mg kg-1) and 11 (1 mg kg-1) group 7-10 (0.6-0.9 mg kg-1) shows significant increase only at week 8-13. There is no significant difference observed in group 1-4 (0.05-0.3 mg kg-1) (Fig. 2b). The group that received 1 mg kg-1 of BPA showed the highest level of MDA-LDL, which consistently rise at week 1-5, 6-11 and week 12-13 with its peak at week5 of exposure (Fig. 2a). Another group that showed a high level of MDA-LDL was the group that received 0.5 mg kg-1 of BPA (Fig. 2a). At all exposure time, the groups exposed 0.6 mg kg-1 of BPA showed a gradual dose-dependent increase except that in all instance the group that received 0.8 mg kg-1 of BPA showed a decline in MDA-LDL relative to that of 0.7 mg kg-1. The study revealed a time-sensitive effect (Fig. 2b). The observed effect was time-dependent except at the test group of 0.2 and 1 mg kg-1 (Fig. 2b).
 |
||||
| |
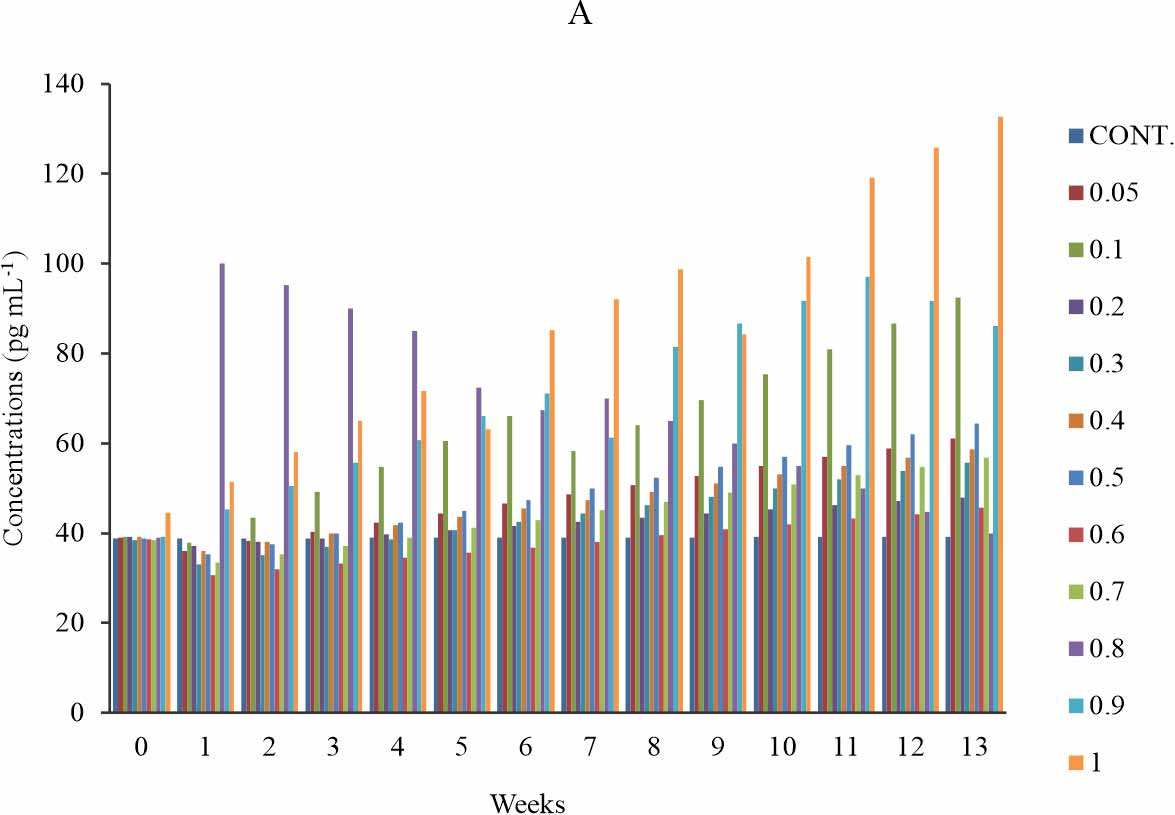
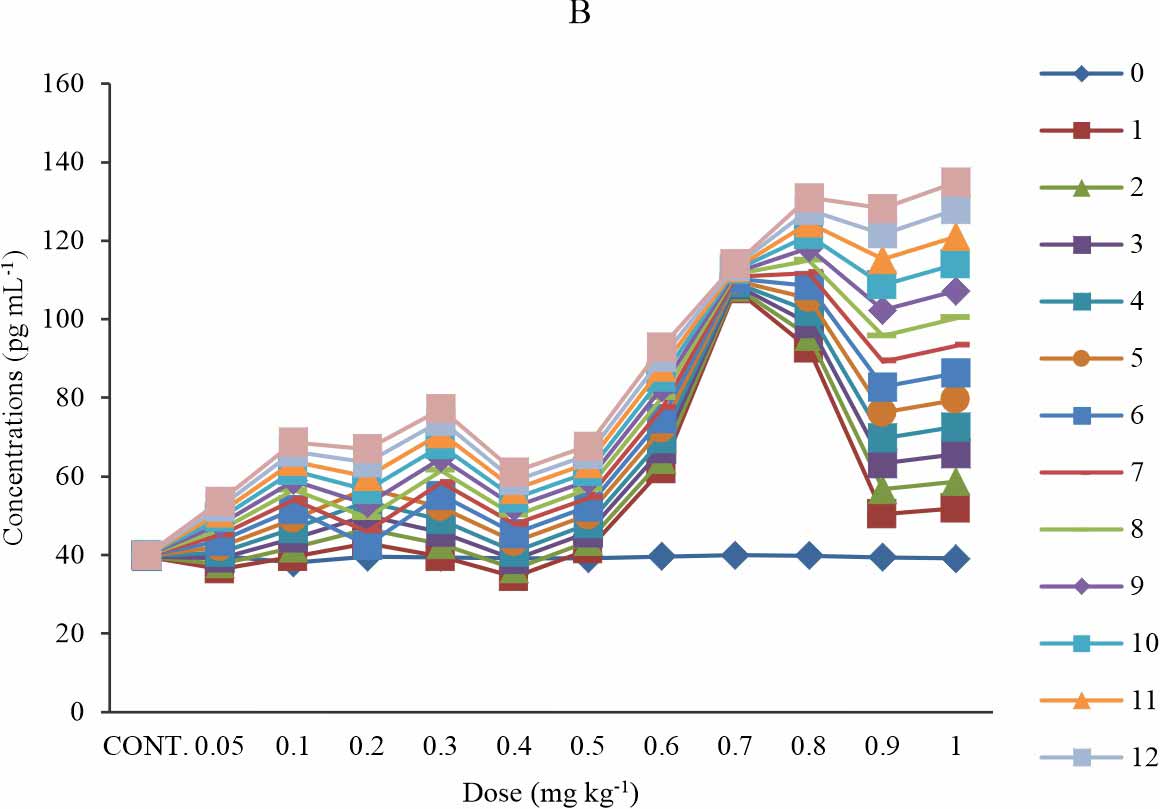
Carboxymethyllysine modified LDL (CML-LDL): There is a significant increase in the CML-LDL level in groups 2 (0.1 mg kg-1), 6 (0.5 mg kg-1), 9-11 (0.8-1 mg kg-1), when compared with the control at p≤0.05 (Fig. 3a). At week 5-13 of exposure to BPA, the groups exposed to 0.2-0.5 mg kg-1 showed a dose dependent increase in carboxymethyllysine modified LDL concentration (Fig. 3a). Group 3-8 (0.2-0.7 mg kg-1) at week 1-3, showed a non-significant decrease compared to the control and week 0; while weeks 7-13 shows a significant increase (Fig. 3b). At group 9 (0.8 mg kg-1), the concentration of carboxymethyllysine modified LDL decreases with the time of exposure while other exposure groups showed increases in the carboxymethyllysine modified LDL level with a time of exposure (Fig. 3b) except at group 11 (1 mg kg-1) where there is a non-systematic response to the effect of BPA (Fig. 3b). The observed effect was time-sensitive (Fig. 3b).
 |
||
| |
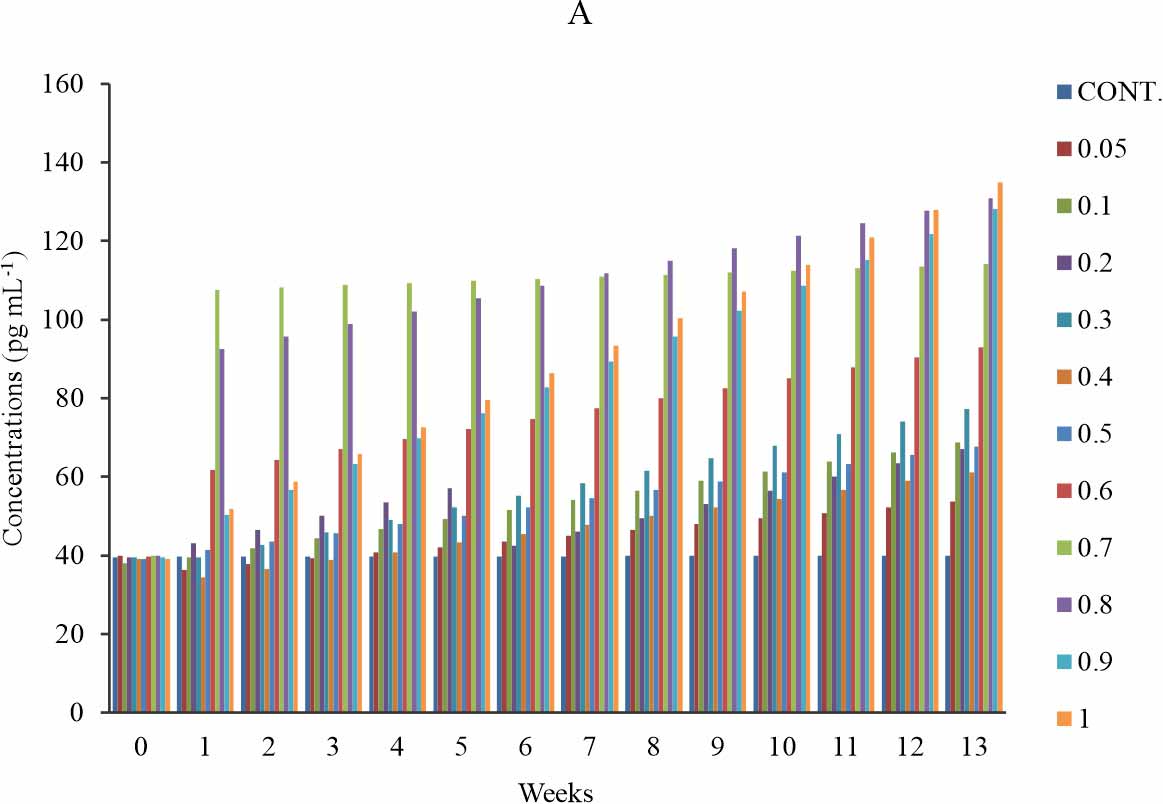
4-HYDROXYNONENAL MODIFIED LDL (HNE – LDL):
There is a significant increase in the HNE-LDL level when compared with the control at p≤0.05. Initially, there was no significant observed for group 1-6 (0.05-0.5 mg kg-1) at week 1-4. The rise was more pronounced in week 13 for the entire exposure groups (Fig. 4a). At week 1-11, the test groups that received 0.4-0.8 mg kg-1 of BPA showed a dose-dependent increase in the serum concentration of HNE-LDL, except at week 1-6, where the that received 0.8 mg kg-1 showed a decline in the HNE-LDL level relative to that of 0.7 mg kg-1 (Fig. 4a) also, it was observed that the test group exposed to 0.9 mg kg-1 of BPA showed a lower level of HNE-LDL relative to that of 1 mg kg-1 group (Fig. 4a). At week12-13, the observed dose-dependent effect extends to 1 mg kg-1 with a slight decline by the group that received 0.9 mg kg-1 relative to that of 1 mg kg-1 and in all the group that received 0.9 mg kg-1 of BPA showed lower HNE-LDL level relative to that of 1 mg kg-1 (Fig. 4a). The observed effect was time-dependent except at test group 0.2 mg kg-1 and time-sensitive except at 0.7 mg kg-1 of BPA exposure group (Fig. 4b).
 |
| |
DISCUSSION
In the current study, BPA increased Reactive Oxygen Species (ROS) production as assessed by the measurement of the end product of lipid peroxidation such as the oxidized lipids such as OXPL-LDL, MDA-LDL, CML-LDL, and HNE-LDL were high. These results are in agreement with the previous studies. Abedelhaffeza et al.16 showed an increase in lipid peroxidation. Ansoumane et al.17 reported an increase in Lipid Peroxidation (LPO). BPA induced oxidative stress in the heart withan increase in MDA levels, hepatic damage and mitochondrial dysfunction18,19. BPA enhances the generation of ROS and reduction of antioxidant defences leading to an aggravated state of oxidative stress. Prolonged exposure to BPA can trigger pathological conditions at extremely low levels thus the demand for its prohibitionin the use of consumers products. Increased lipid peroxidation indicates an increased oxygen free radical generation which compromises the integrity of mitochondrial function20. It has been reported that BPA exposure results in augmentation of oxidative stress, with increased levels of lipid peroxidation and reactive oxygen species and depletion of the antioxidant defence system21. Lipids such as phospholipid and other types of lipids found in cells especially as components of the plasma membrane and organelle membranes are another major target for the generated ROS, free radicals and Oxidative stress22. Lipid oxidation derived products assayed as oxidized lipid, the study showed that the serum levels of the 4 oxidized lipid products were high. They include oxidized phospholipid LDL, MDA-LDL, CML-LDL and HNE- LDL. The consequence of these findings is impaired membrane functions, inactivate membrane-bound receptors and enzyme and increase cell membrane permeability, leading to the opening of the mitochondrial permeability transition pore that may cause the release of pro-apoptotic molecules to the cytosol17. In addition to the oxidized lipids, depletion of reduced glutathione (GSH) also facilitates the opening of the mitochondrial permeability transition pore. These molecules are usually more reactive than the initial molecules they were formed from and present a range of pathological effects, including increased vascular permeability, Low-Density Lipoprotein (LDL) oxidation and enhancing oxidative stress20. Oxidation of free radical is the cause of degradation of fatty acids and their esters in cellular membranes and lipoproteins,leading tothe development of severe pathological conditions. The damage on the cellular membrane by lipid peroxidation of membranes fatty acid22 andthe oxidative damage to lipoproteins, specifically LDL, plays a role in some diseases conditions, which includes cardiovascular diseases, arthritis, dementia and metabolic syndrome. High oxidized LDL concentrations have been linked to an increased cardiovascular disease risk20, promotes endothelial cell damage, and are chemotactic for leukocytes and endocytosed by macrophages20.
Oxidative damages to lipids have seriously deleterious and are concomitant with the oxidative damages to proteins which are the vehicle for oxidative damage on cells because they are often catalysts; hence, the effect of damage to one molecule is greater than stoichiometric. ROS leading to protein oxidation include radical species such as superoxide, hydroxyl, hydroperoxyl, and non-radical species such as hydrogen peroxide (H2O2), singlet oxygen (1O2) and peroxynitrite (ONOO_)23.
CONCLUSION
BPA as an endocrine-disrupting chemical interferes with lipid metabolism and balance, thereby disrupting the lipid homeostasis that might lead tomembrane dysfunction and energy homeostasis disorders. At very low doses, BPA causes permanent alterations of lipid in the cells that precede cancer, a wide range of metabolic diseasesand other diseasepathologies. The increased BPA exposure and its range of target biomolecule emphasize the need for a comprehensive evaluation and assessment of the effects of BPA exposure.
SIGNIFICANCE STATEMENT
BPA is associated with many health problems and diseases that are on the rise in the human population, including cancer and infertility. It induces oxidative stress, provokes antioxidant activity and interferes with lipid metabolism and energy balance. This study will help the researcher to uncover the critical areas of Bisphenol-A toxicity that many researchers were not able to explore. Thus a new theory on lipid oxidation due to BPA exposure may be arrived at.
REFERENCES
- Ehrlich, S., A.M. Calafat, O. Humblet, T. Smith and R. Hauser, 2014. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA, 311: 859-860.
- Liao, C. and K. Kannan, 2011. Widespread occurrence of bisphenol a in paper and paper products: Implications for human exposure. Environ. Sci. Technol., 45: 9372-7379.
- Braun, J.M., A.E. Kalkbrenner, A.M. Calafat, K. Yolton, X. Ye, K.N. Dietrich and B.P. Lanphear, 2011. Impact of early-life bisphenol a exposure on behavior and executive function in children. Pediatrics, 128: 873-882.
- He, Y., M. Miao, L.J. Herrinton, C. Wu, W. Yuan, Z. Zhou and D. Li, 2009. Bisphenol-A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ. Res., 109: 629-633.
- Mikołajewska, K., J. Stragierowicz and J. Gromadzińska, 2015. Bisphenol-A – Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health, 28: 209-241.
- Chinenye E.O and C.E. Francis., 2017. Bisphenol a (bpa) increases blood triglycerides and low density lipoproteins in albino wistar rats. J. Exp. Res., 5: 24-27.
- Miyawaki, J., K. Sakayama, H. Kato, H. Yamamoto and H. Masuno, 2007. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb., 14: 245-252.
- Mourad, I.M. and Y.A. Khadrawy, 2012. The sensetivity of liver, kidney andtestis of rats to oxidative stress induced by different doses of bisphenol A. Life Sci. Pharmacol. Res., 2: 19-28.
- Invernizzi, P., 2013. Liver auto-immunology: The paradox of autoimmunity in a tolerogenic organ. J. Autoimmunity, 46: 1-6.
- Alonso-Magdalena, P., E. Vieira, S. Soriano, L. Menes, D. Burks, I. Quesada and A. Nadal, 2010. Bisphenol-A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect., 118: 1243-1250.
- Oguazu, C.E., C.E. Francis, I.U. Kingsley and A. Benedeth, 2015. Bisphenol a exerts a transient perturbation of liver function in wistar albino rats at acute and sub-chronic exposure doses. J. Pharm. Sci. Bioscientific Res., 5: 274-278.
- Lang, I.A., T.S. Galloway, A. Scarlett, W.E. Henley, M. Depledge, R.B. Wallace and D. Melzer, 2008. Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. J. Am. Med. Assoc., 300: 1303-1313.
- Park, Y.J., W.S. Kwon, S.A. Oh and M.G. Pang, 2012. Fertility-related proteomic profiling bull spermatozoa separated by percoll. J. Proteome Res., 11: 4162-4168.
- Ezeonu, F.C., E.O. Chinenye, I.U. Kingsley and A. Benedeth, 2015. Bisphenol a causes blood electrolyte imbalance and upsets kidney functions in albino wistar rats. J. Pharm. Sci. Bioscientific Res., 5: 547-550.
- Eid, J.I., S.M. Eissa and A.A. El-Ghor, 2015. Bisphenol-A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J. Basic Applied Zool., 71: 10-19.
- Abedelhaffez, A.S., E.A.A. El-Aziza, M.A.A. Aziz and A.M. Ahmed, 2017. Lung injury induced by Bisphenol-A: A food contaminant, is ameliorated by selenium supplementation. Pathophysiology, 24: 81-89.
- Kourouma, A., C. Quan, P. Duan, S. Qi, T. Yu, Y. Wang and K. Yang, 2015. Bisphenol-A induces apoptosis in liver cells through induction of ROS. Adv. Toxicol.
- Moon, M.K., M.J. Kim, I.K. Jung, Y.D. Koo and H.Y. Ann et al., 2012. Bisphenol-A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J. Korean Med. Sci., 27: 644-652.
- Asahi, J., H. Kamo, R. Baba, Y. Doi and A. Yamashita et al., 2010. Bisphenol-A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci., 87: 431-438.
- Aboul Ezz, H.S., Y.A. Khadrawy and I.M. Mourad, 2015. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology, 67: 145-155.
- Eid, J.I., S.M. Eissa and A.A. El-Ghor, 2015. Bisphenol-A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J. Basic Applied Zool., 71: 10-19.
- Shu, L., A. Vivekanandan-Giri, S. Pennathur, B.E. Smid, J.M.F.G. Aerts, C.E.M. Hollak and J.A. Shayman, 2014. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int., 86: 58-66.
- Nandi, D., R.C. Patra and D. Swarup, 2005. Effect of cysteine, methionine, ascorbic acid and thiamine on Arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology, 211: 26-35.
How to Cite this paper?
APA-7 Style
Oguazu,
C.E., Ezeonu,
F.C., Azuka,
A.B., Ikimi,
C.G., Onuabuchi,
A.N., Nwobodo,
V.O., Dike,
C.C. (2021). Bisphenol-A Exposure Causes Increased Oxidation of LDL and Its Abduct in Rats. Asian Journal of Emerging Research, 3(2), 124-128. https://doi.org/10.3923/ajerpk.2021.124.128
ACS Style
Oguazu,
C.E.; Ezeonu,
F.C.; Azuka,
A.B.; Ikimi,
C.G.; Onuabuchi,
A.N.; Nwobodo,
V.O.; Dike,
C.C. Bisphenol-A Exposure Causes Increased Oxidation of LDL and Its Abduct in Rats. Asian J. Emerg. Res 2021, 3, 124-128. https://doi.org/10.3923/ajerpk.2021.124.128
AMA Style
Oguazu
CE, Ezeonu
FC, Azuka
AB, Ikimi
CG, Onuabuchi
AN, Nwobodo
VO, Dike
CC. Bisphenol-A Exposure Causes Increased Oxidation of LDL and Its Abduct in Rats. Asian Journal of Emerging Research. 2021; 3(2): 124-128. https://doi.org/10.3923/ajerpk.2021.124.128
Chicago/Turabian Style
Oguazu, Chinenye, E., Francis C. Ezeonu, Anajekwu B. Azuka, Charles G. Ikimi, Ani N. Onuabuchi, Valentine O. Nwobodo, and Charles C. Dike.
2021. "Bisphenol-A Exposure Causes Increased Oxidation of LDL and Its Abduct in Rats" Asian Journal of Emerging Research 3, no. 2: 124-128. https://doi.org/10.3923/ajerpk.2021.124.128

This work is licensed under a Creative Commons Attribution 4.0 International License.