Removal of Industrial Dye via Biohybrid Composites Characterized by FTIR and SEM
| Received 20 Dec, 2020 |
Accepted 05 Mar, 2021 |
Published 18 Jun, 2021 |
ABSTRACT
Background and Objective: Dyes that discharge from the industries are very toxic even at very minimum concentrations. Consequently, the exclusion of the dyes is very essential from waste water. Therefore, this study was designed to remove the synthetic dye by using bio-hybrid composites. Materials and Methods: Bio-hybrid composites MUS-ZnO-PANI (mustard zinc oxide polyaniline) and MUS-ZnO-PPy (mustard zinc oxide polypyrrole) were synthesized for dye exclusion via using the adsorption method. An experiment was performed under different optimized conditions. To find surface morphology, functional groups, active binding and thermal stability FTIR and SEM techniques were used. Results: For all types of biosorbents maximum removal of AAR-5 (Act acid red 5) dye observed at 2 pH and the level of dosage was 0.05 g/50 mL. While the equilibrium achieved in 60-90 min with maximum absorption capacity. A suitable temperature to exclude the dye was observed and that was 35°C. Conclusion: After this study, it was concluded that the elimination of the dye effluent from waste water is important to keep the environment healthy. The results of this study proved that the adsorption method is an economical technology for the exclusion of dye from waste water.
INTRODUCTION
Today, water quality is facing many critical problems. As, for the sustainability of life, pure water is essential for living organisms. Due to the rapid increase in urbanization and industrialization water scarcity become a foremost issue among all living organisms1. According to the estimation of 2025, 50% of the world population are suffering from water shortage2. Moreover, the high utilization of fresh water in different sectors is a major cause of pollution in waste water.
Many industries are involved in the use of different synthetic dyes. Synthetic dyes have an aromatic structure due to which they are biologically non-degradable. The presence of a trace amount of dye in effluents is undesirable and highly visible. Annually, a million tons of the dyes produced, used in textile, leather, cosmetics and thousand tons of dyes released into water resources cause aquatic problems such as high colouring, biological toxicity, high COD (chemical oxygen demand), etc. So the release of dyes in water is a major source of esthetic pollution perturbation and eutrophication in water. Dye effluent contains dangerous chemicals that are toxic, mutagenic and carcinogenic for living things and causes diseases such as nausea, ulceration, haemorrhage and severe damage to the liver, kidney, brain, reproductive system, and CNS. Probably dyes, lead to many environmental problems3. So, it is very important to treat the polluted water before discharging it in large water reservoirs. Several technologies employed for the elimination and degradation of waste water, such as filtration, artificial UV radiation adsorption, osmosis, biodegradation, ozonation, chlorination and solar pasteurization. These methods are costly, intensive, requires high energy, modified equipment, and skilled operators4. Few drawbacks of these techniques include the requirement of a large number of chemicals and proper handling for a long time. Moreover, secondary pollutants are generated during the treatment which requires a long process for removal. Therefore, there is a need to develop an effective, operational and lost cost system for the removal of waste water pollutants and for improving the water quality hybrid materials are considered best for the purification of waste water. Hybrid technology is a combination of multi-disciplinary technologies such as nanotechnology, information technology and biotechnology resulting in advanced scientific knowledge. At the nanometer scale, hybrid materials are extensively studied in different fields of life science such as power generation, eco electronics, polymer engineering, and fine chemistry5. Therefore, biohybrid materials are used at a high scale for the treatment of waste water.
Polyaniline is an imperative conducting polymer that has been achieved importance just due to the divergent uniqueness such as protonation reversibility, excellent electrochemistry, redox recyclability, and stability. Polyaniline is such a type of adsorbent which is cost-effective, and have a high-quality adsorption capacity for dyes. Polyaniline has the protonated amine group that shows the electrostatic interaction among anionic contaminants. Polypyrrole is conducting polymer and studied generally because of its environmental stability and high thermal capability6. Polyaniline composites have become remarkable in the recent era due to the low cost, easy preparation, physiochemical properties, environment stability and many attractive practical applications7. Composites of the polyaniline are fashioned to improve the adsorption properties just because of the particles of the sole polyethylene which exist in the aggregate appearance in the aqueous solution because it shows a reduced amount of adsorption capacity due to the low kinetic rate and low surface area. Treatment of the waste water and removal of dyes can be done through different methods by using biohybrid composites.
In the present work, it was aimed to synthesize MUS-ZnO-PANI and MUS-ZnO- PPy hybrid composite through doping of the polyaniline and polypyrrole with MUS-ZnO to obtain negative charge composites with a large surface area for adsorption of cationic dye. The PANI and PPy doped with MUS-ZnO composite for cationic dyes are predicted to have discriminatory adsorptive nature. In this study, adsorption of MUS-ZnO-PANI and MUS-ZnO-PPy hybrid composites for the removal of AAR-5 dye from the aqueous solution was observed.
MATERIALS AND METHODS
Study area: This research was conducted for the 18th month (January 2017 to June 2018) in the Environmental Chemistry Lab, Chemistry Department, University of Agriculture Faisalabad, Pakistan.
Materials: The reagents and chemicals such as zinc chloride (ZnCl2), sodium hydroxide, polyaniline, polypyrrole, ammonium persulfate (APS), ferric chloride and hydrochloric acid (HCl) were used in the study. All these chemicals were primarily purchased from Merck (Germany) and Sigma-Aldrich Chemicals Co. (USA) unless otherwise acknowledged and all were of analytical grade. Agro waste or biomass used for the synthesis of the composite were collected from Chakwal Pakistan. Washed with distilled water and then dried afterwards dry plant ground in an agate mortar and stored for further purposes.
Synthesis of biohybrid composites:
Chemical synthesis of MUS-ZnO-PANI biohybrid composite: The MUS-ZnO-PANI hybrid composite was synthesized in two steps:
1st step: The first step was a synthesis of MUS-ZnO
2nd step: MUS-ZnO-PANI composite.
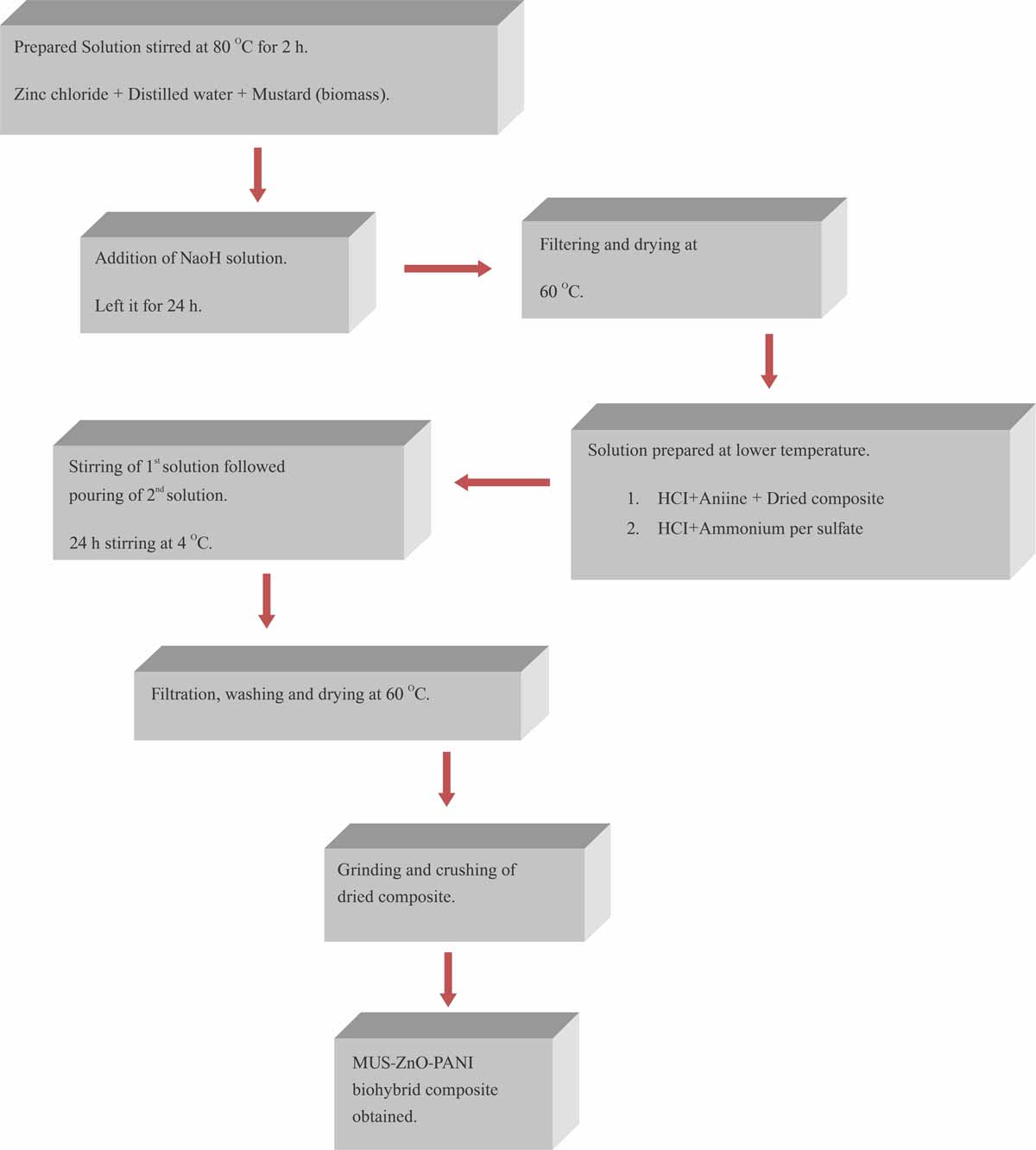
Preparation of Zinc oxide, ZnO: Zinc oxide (ZnO) prepared from Zinc Chloride and Sodium hydroxide following the previously reported method7. In the first step, zinc oxide was prepared through a method of chemical precipitation. 6.25 g zinc chloride dissolved in 50 mL distilled water. In resultant, solution (I) 0.5 g of mustard biomass added. Solution (I) was allowed to stir continuously for 2 hrs at 80°C to accompany the electrostatic interaction of zinc ions onto biomass. Then the solution (I) cooled at room temperature. 25% NaOH solution (II) prepared and added in the solution (I), left for reaction at room temperature for 24 hrs. The resultant precipitate washed with de-ionized water many times to eliminate the excess material and impurities.
Synthesis of ZnO-PANI composites: MUS-ZnO-PANI composite was prepared from aniline HCl and APS oxidant. In the second step, precipitates obtained from the above method were dispersed in 1.02 g aniline and dissolved in 0.5 M HCl of 50 mL [Solution (III)]. Prepared solution (III) sonicated for 30 min via using ultrasonication and further stirred for 2 hrs. Afterwards, 0.2 g of ammonium persulfate oxidant was dissolved in 20 mL of 0.5 mL HCl [Solution (IV)]. Solution (IV) added dropwise in solution (III). Accordingly, reactive vessels kept in an ice bath (0-5°C) with magnetic stirring for 4 hrs and then the mixture allowed to rest for 24 hrs. statically. Obtained greenish-black solid filtered and washed several times with deionized water. Filtered residue collected and dried at 80°C for 12 hrs. The final form of greenish-black solid obtained was designated as MUS-ZnO-PANI hybrid composite as shown in Fig. 1 and 2 depicted the whole procedure.
 |
||||||||||||||||||||||||||||||||||||||||
| |
 |
||||||||||||||||||||||||||||||||||||||
| |
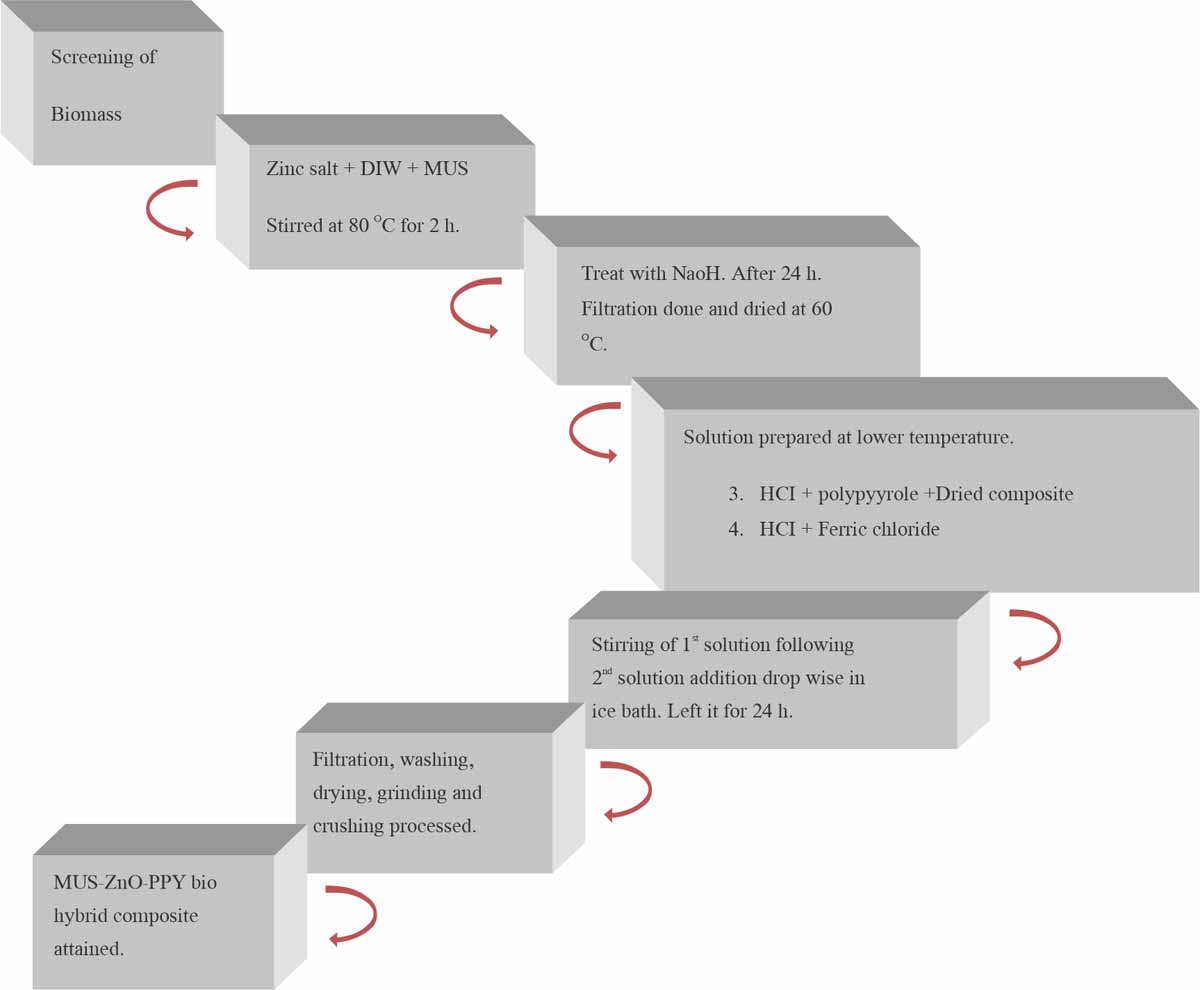
Chemical synthesis of MUS-ZnO-PPybiohybrid composite: Mustard zinc oxide polypyrrole biohybrid composites were synthesized in the same manner as mustard zinc oxide polyaniline biohybrid composite. Differs only in one way as in a second step after synthesis of MUS-ZnO, ferric chloride was added instead of ammonium persulfate and polypyrrole was added in place of polyaniline7 as shown in Fig. 3 and 4 depicted the whole procedure.
 |
||||||||||||||||||||||||||||||||||||
| |
 |
||||||||||||||||||||||||||||||||||
| |
Adsorption experiments: An experiment of batch studies8 was conducted for comparing the capacities of biosorption for biosorbents in biocomposite plus native forms. Different parameters optimize as pH, biosorbent dose, contact time, temperature and concentration of dye for the elimination of dyes conceded via standard approach. Conical flasks of 250 mL containing the solution of the dye of 50 mL of known concentration, pH and dose, shake in the orbital shaking incubator at the speed 120 rpm. Using 0.1 M NaOH and HCl solution pH was adjusted. The experiments were repeated and values obtained were symbolized as the mean±SD. With time centrifugation process was performed at the speed of 500 rpm for 20 min after taking out the sample and the concentration of the remaining solution of dye was determined via UV-Vis spectrophotometer (Shimadzu, Japan). The effect of the presence of the different electrolytes as well as surfactants was examined.
$$ \mathrm{qe}=\frac{\left(\mathrm{C}_{\mathrm{o}}-\mathrm{C}_{\mathrm{e}}\right) \times \mathrm{V}}{\mathrm{W}} $$Where,
C0= Concentration of dye (mg L-1)
Ce= Equilibrium concentration of dye (mg L-1)
V = Solution volume (L)
W = Mass of the biosorbents (g)
Different parameters were optimized under different conditions. The pH study was done by changing the pH range from 2-11. Study of dose level was observed by changing the amount of the biosorbent dose 0.05-0.3 g/ 50 mL solution of dye 50 mg L-1 concentration. Dye concentration study was performed by changing the amount of dye from 10-200 mg L-1. To detect the most suitable temperature for dye absorption different temperature ranges were used (range: 30-71°C). The effect of the contact time observed via changing the contact duration from 5-90 min. Standard conditions during optimization of the above-mentioned parameters kept constant such as particle size: 300 μ m; shaking speed: 120 rpm.
Characterization
FTIR: FTIR technique was carried out to investigate the active functional groups of biosorbents. FTIR spectra showed different peaks at different intervals with elevated crust and troughs which indicate the biosorbents complex nature.
SEM: SEM analysis was accomplished to observe the microstructure with the addition of AAR-5 Dye. SEM facilitates scrutinizing the biohybrid composites at the micro-level. Three different biohybrid composites were examined to distinguish the behaviour of these samples after AAR_% dye absorption. Samples were placed on a sample holder coated with silver paint and gold to prevent the charge interaction created between sample and electron absorbed by the sample. An accelerating voltage of 5 kilo volts was applied to attain desired magnification results.
RESULT AND DISCUSSION
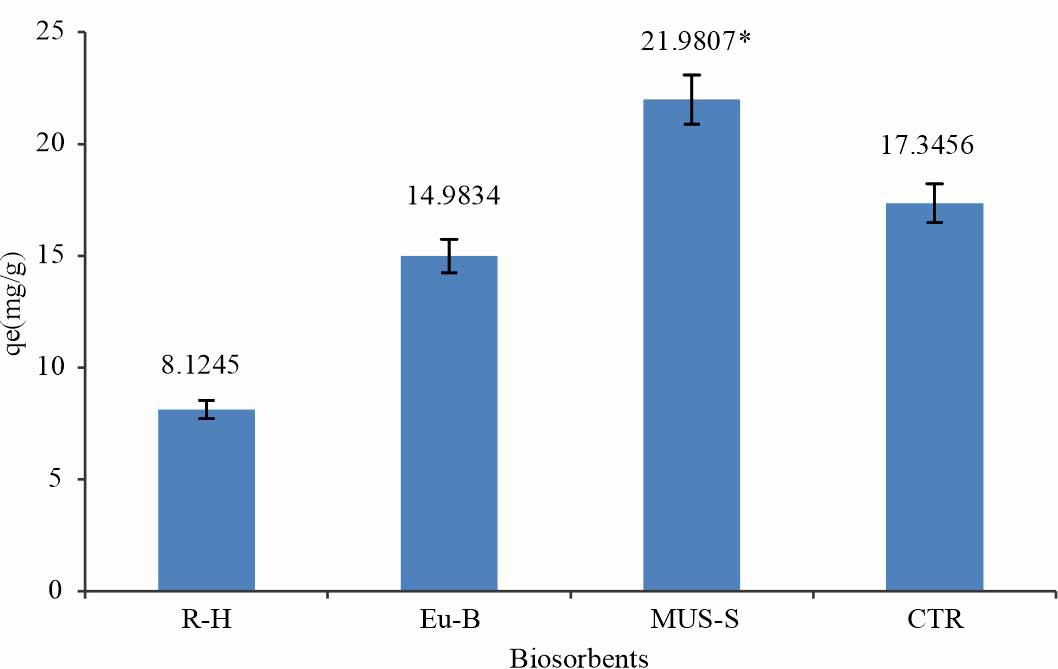
Screening of agrowaste: To select out the most efficient biosorbent screening of the agro-waste (rice husk, eucalyptus bark, mustard stem and citrus) for acid dye was performed to attain maximum dye elimination. The result revealed that various types of agrowastes gave unusual biosorption for acid dye like mustard stem, citrus, rice husk and eucalyptus bark have different biosorption capacity for AAR-5as shown in Fig. 5. Thus, the most efficient biosorbent Mustard Stem (MUS-S) obtained from screening8 with the acid dye used for the further study.
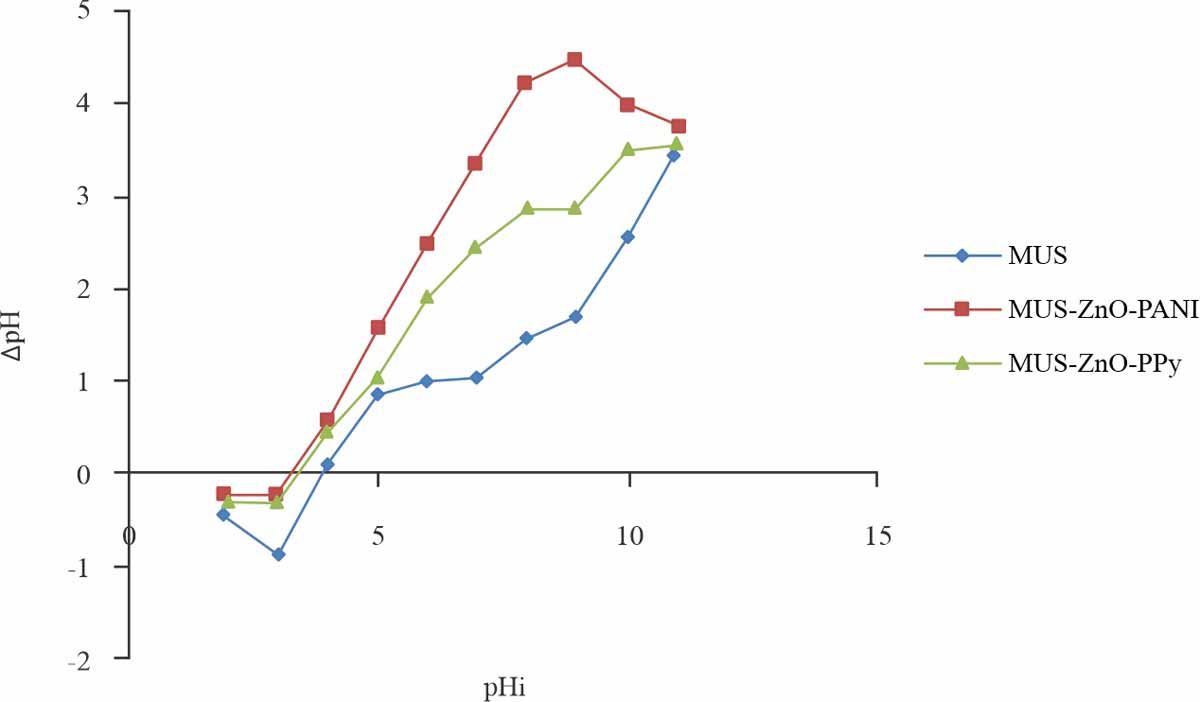
Determination of point zero charges: Biosorption capability of the biosorbent investigated through significant factor point zero charges (pHpzc). When the charge on the biosorbent surface is zero, then the situation called point zero charges. pH ˃ pHpzc suitable condition for cations biosorption while pH ˂ pHpzc condition is favourable for anions biosorption. pHpzc plays a major role to find the charge on the surface that either is positive or negative9. pHpzc of MUS, MUS-ZnO-PANI and MUS-ZnO-PPy showed great affinity in the acidic range for dye adsorption. Results inferred that pH values of biosorbents have a positive charge due to protonation of the functional group of surfaces and for anionic dye electrostatic interaction showed while negative created on the surface of biosorbents above these values of pH as shown in Fig. 6.
Adsorption studies
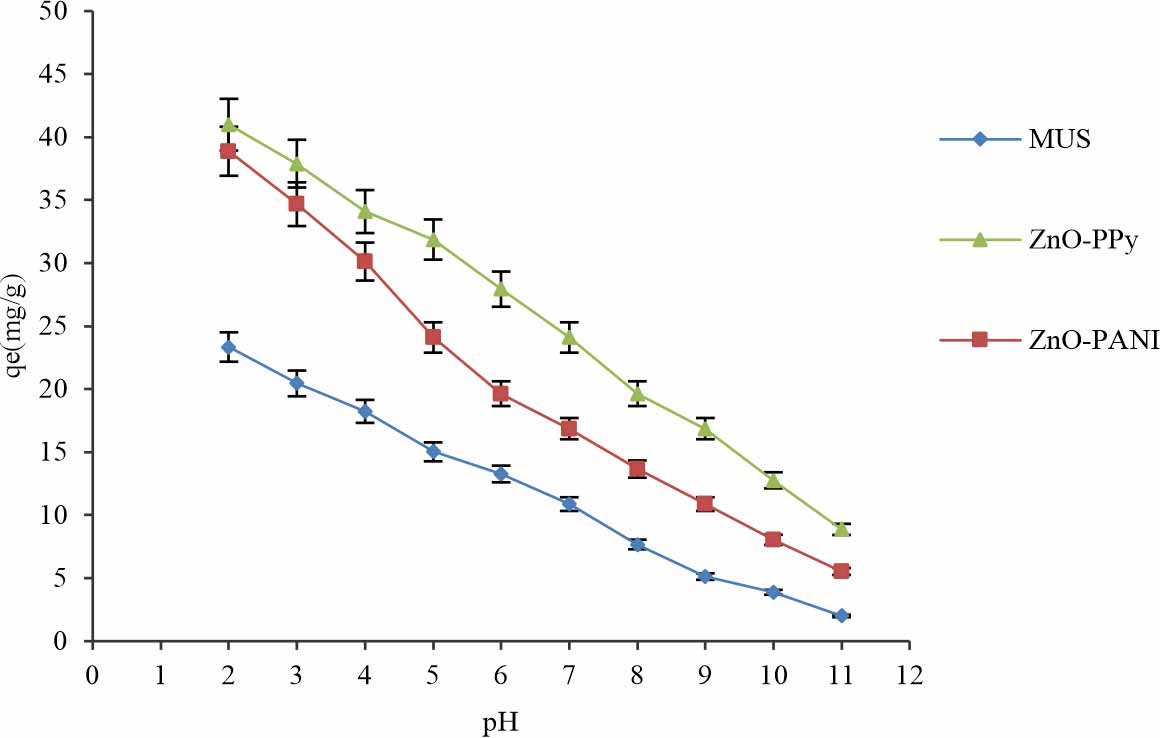
Effect of pH: pH determines the basicity or acidity of an aqueous solution. pH is a significant factor that affects the surface charge, speciation and the dissociation of the active sites of functional groups adsorbent that explain the adsorption performance of dyes and metals. For that reason, the effect of the pH on speciation and sorption is valuable in the considerate adsorption process of electrostatic interaction. The pH of the solution with different composites investigated by changing the value of pH from 2-11. Colour change was observed at 1 pH. The maximum values of biosorption capacity for MUS (23.354 mg g-1), MUS-ZnO-PANI (38.8792 mg g-1) and MUS-ZnO-PPy (40.9872 mg g-1) biocomposites were observed at 2 pH respectively in Fig. 7. Therefore, pH is a significant environmental factor that affects the biosorption process of acid dyes through different biosorbents and removal of the acid dye was maximum in the acidic environment.
In this study best dye removal obtained at 2 pH, which shows similar behaviour with another study of Pandimurugan and Thambidurai7 that hybrid composites for exclusion of MB dye observed at pH range 3-11 and adsorption of dye increase with the increase in initial pH.An increase in pH causesan increase in the negative charge surface through the absorption of hydroxyl (OHˉ) ions. So, surface sites of negative charge on MUS-ZnO-PPy and MUS-ZnO-PANI preferred the adsorption for acidic dye due to electrostatic attraction. Moreover, the effect of pH on electrostatic properties and adsorption properties between dye and adsorbent play important role in the adsorption process. Investigations showed that removal of reactive dye achieved at 2 pH in the range of acidic media10. The highest electrostatic interaction found in the acidic conditions because of the production of the positive charge surface on biosorbent surfaces due to the higher H+ concentration of ions. Moreover, in the environment of basic media, electrostatic repulsion was dominant that cause a reducing effect of dye removal.
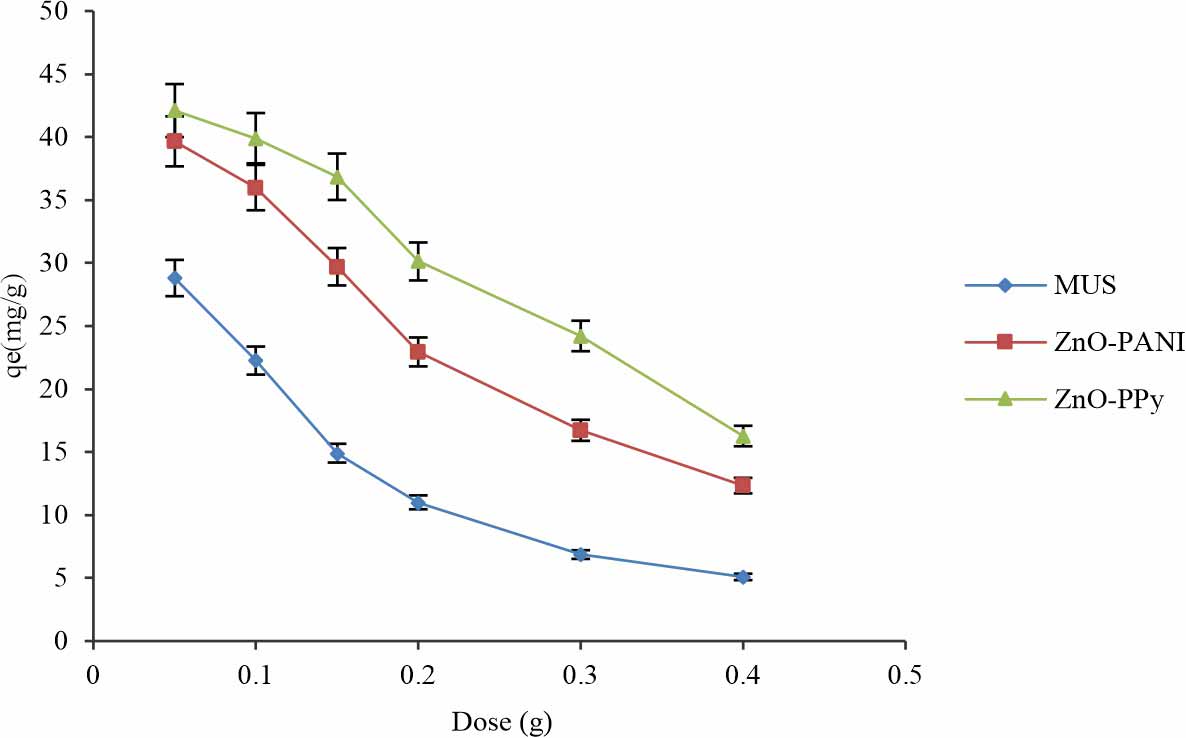
Effect of dose: Biosorbent dosage is an essential parameter to investigate the biosorption capability of certain sorbate at constant experimental conditions10. For optimization of the dosage of adsorbent in dye solution time, pH, shaking speed and concentration kept constant. Dosage effect on the degradation of the dye was studied by changing the dye concentration from 0.05 0.1, 0.15, 0.20, 0.30 and 0.40 g. The effect of biocomposites of acid dye studied under fixed operating conditions 50 mg L-1 concentration of dye, optimum pH, 120 rpm shaking and 30°C temperatures. The efficiency of degradation was increased with an increase in the concentration of adsorbent. Degradation of acid dye increased due to an increase in adsorption sites and surface area.
Results showed that 0.05 g was the best adsorbent concentration. According to the results, it was observed that the absorption capacity of all biosorbents for MUS (28.809 mg g-1), MUS-ZnO-PANI (39.6541 mg g-1) and MUS-ZnO-PPy (42.0987 mg g-1) biocomposites with the addition of the dose of biosorbent 0.05 to 0.40 g/50 mL in the solution of dye were observed at 2 pH in Fig. 8. It was investigated that the highest acid dye adsorption was obtained at the lowest dose of biosorbent. A decrease in biosorption capability at high doses of biocomposites could be due to the overlapping of the binding surface of active sites that cause a reduction in surface area that binds the anions of dye. According to Pandimurugan and Thambidurai7 MB dye removal best at biosorbent dose of 0.05 g/50 mL which showed similar behaviour with the current study in the removal of AAR-5 dye. Investigations showed a reduction in the absorption capability due to an increase in the dose of biosorbent in the dye solution. The reason behind such type of behaviour is due to the large surface area and lower biosorbent dose which causes more collision in dye molecules11.
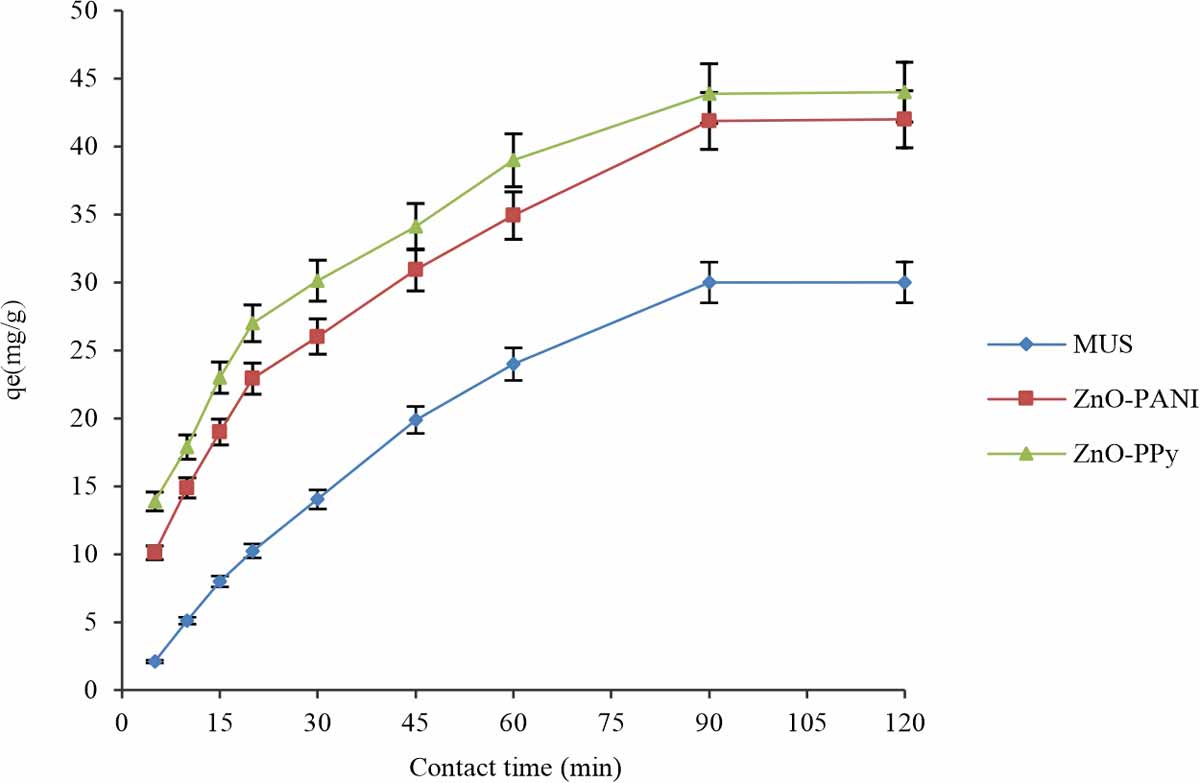
Effect of contact time: The effect of the contact time for Sorbent and sorbate to remove the acid dye via various biosorbents observed by performing the experiments of biosorption in contact periods 5-90 min12. Effect of contact periods (5-90 min) for different biosorbents (MUS, MUS-ZnO-PANI and MUS-ZnO-PPy) on biosorption of the acid dye using optimum condition for a dose of biosorbents, pH, shaking speed 120 rpm, 50 mg L-1 concentration of dye, 30°C temperature and particle size 300 μm while keeping another parameter variable e.g contact time. Results have shown that biosorption of acid dye through different composites was very fast in the first 45 min and then slows down and attains equilibrium in 60 min. The maximum values for biosorption capacity of biosorbents were MUS (29.9876 mg g-1), MUS-ZnO-PANI (45.8769 mg g-1) and MUS-ZnO-PPy (43.8967 mg g-1) obtained in 60 min and then equilibrium achieved in Fig. 9. Maximum removal of acid dye at 60 min attained and 60 min was an optimum contact period for removal of acid dye. To remove the MB dye experiments were performed and investigated that contact time for removal of methylene blue (MB) dye was increased till 60 min and then attained equilibrium13.When equilibrium was established no further increase in the capacity of adsorption was observed. At the start of the adsorption process, biosorption rate was very fast and then decrease with time unless equilibrium reached. The rapid rate in beginning can be due to the accessibility of the active sites in large number for biosorption and a lower rate of biosorption may be because of their diffusion with time after that equilibrium achieved.
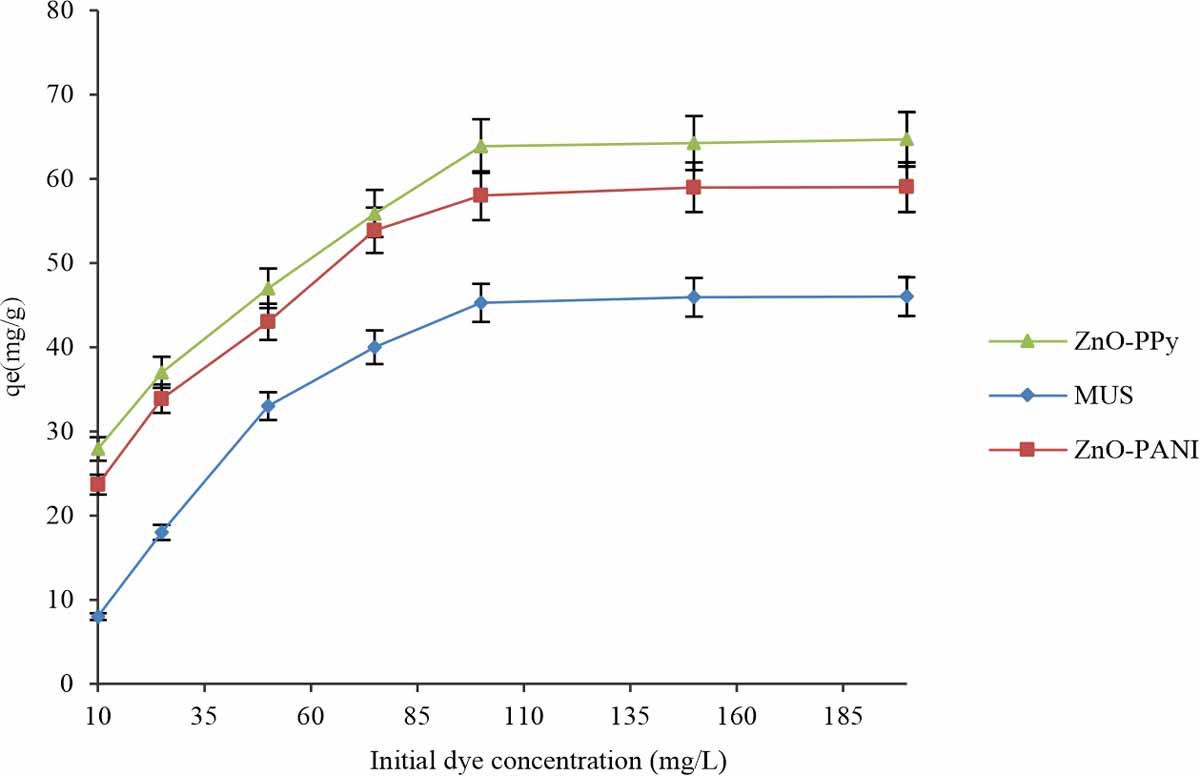
Effect of concentration of dye: The effect of initial dye concentration is very important for the biosorption process. Batch experiments conducted to investigate the dye concentration (range: 10-200 mg L-1) effect on biosorbents for acid dye removal at optimum conditions such as pH, equilibrium contact time, 0.05 g/50 mL biosorbent dose, 30°C temperature and 120 rpm shaking. Results indicate that there was an increasing trend in biosorption capability of different biosorbents via increasing the dye concentration in Fig. 10. The reason behind such type of trend may be due to providing the driving force via initial dye concentration for slow down the mass transport resistance in the aqueous and solid phase and equilibrium achieved because of the establishment of the dynamic balance in dye concentration and the surface of biosorbent14. The same effect for the removal of MB dye was observed that experiments conducted with a variable initial dye concentration of MB in biohybrid composite SW-ZnO-PANI7. At initial concentration, dye completely absorbed, but at the maximum concentration of dye, the dye did not completely absorb. Therefore, such a condition indicates that a saturation limit is there above which removal of dye is not possible.
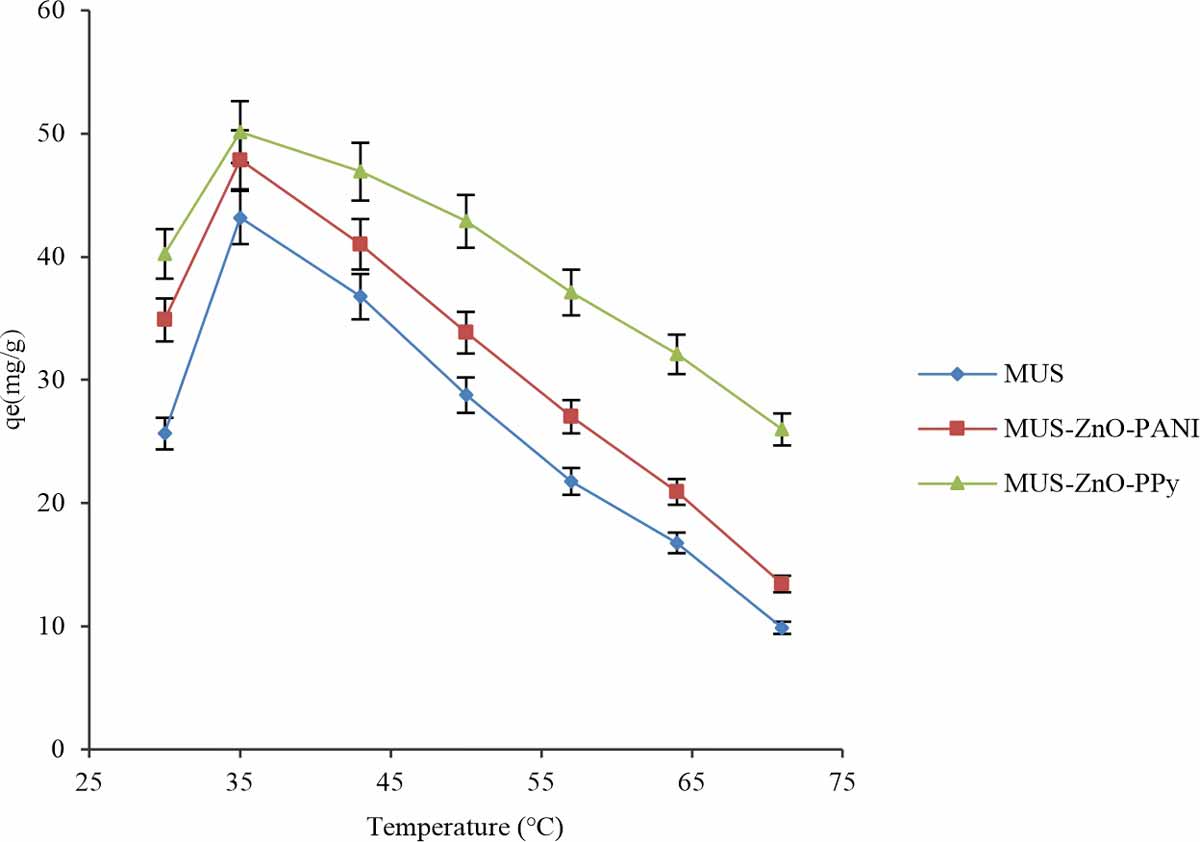
Effect of temperature: Textile industries liberate sewage at high temperature. Therefore, the temperature has a vital role in the process of biosorption and also a significant factor in the designing of the sorption system. The effect of the different temperatures (range: 30-71°C) on different biosorbents was checked out to eliminate the acid dye from the aqueous solution by keeping fixed experimental conditions.
Results showed a significant reduction in the biosorbents biosorption capability for removal of acid dye that shows exothermic nature for all biosorption process. The highest biosorption capacity for all biosorbents achieved at 43°C for removal of acid dye in Fig. 11. The decline in biosorption of acid dye at utmost temperature may be owed to the breakdown of the sorption forces that is dependable for biosorption dye on biosorbent surface8. The same effect was observed for the exclusion of Novacron Orange P-2R using bagasse15.
Effect of temperature was ranging from 30-60°C for the elimination of reactive dye by experimenting with optimum pH and dose16. They observed that increase in temperature cause diverse effects while at lower temperature removal of dye was very effective.
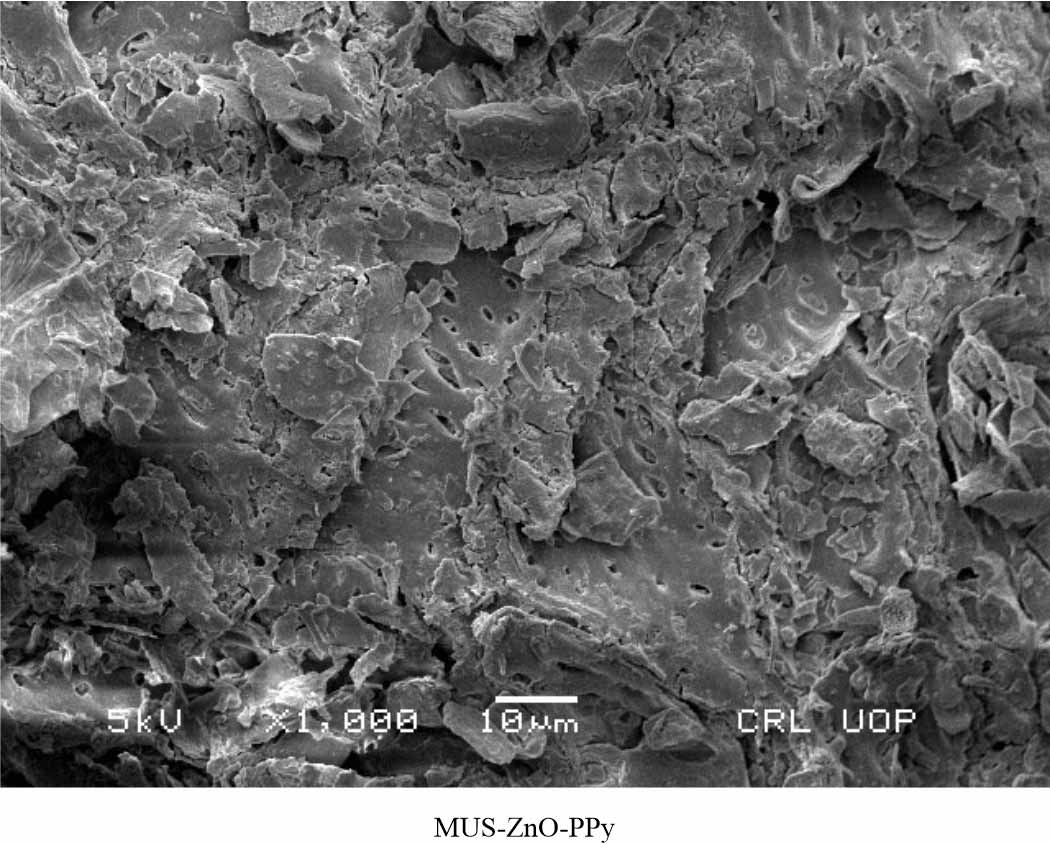
SEM analysis: Scanning Electron Microscopy was carried out on the native mustard compound by using the standard 5 kilovolts treated with 100μm sample amount under 200 x resolutions as shown in Fig. 12. While SEM analysis of biohybrid composites MUS-ZnO-PANI and Mus-ZnO-PPy was performed on 10 μm samples by applying 5 kilo volts which can be observed clearly under 1000 x resolutions as shown in Fig. 13 and 14, respectively. As both the composite reduced the effect of dye, but Mus-ZnO-PPy composite showed effective results as compare to MUS-ZnO-PANI composite and completely decolourize the dye solution via showing excellent biosorption capacity.
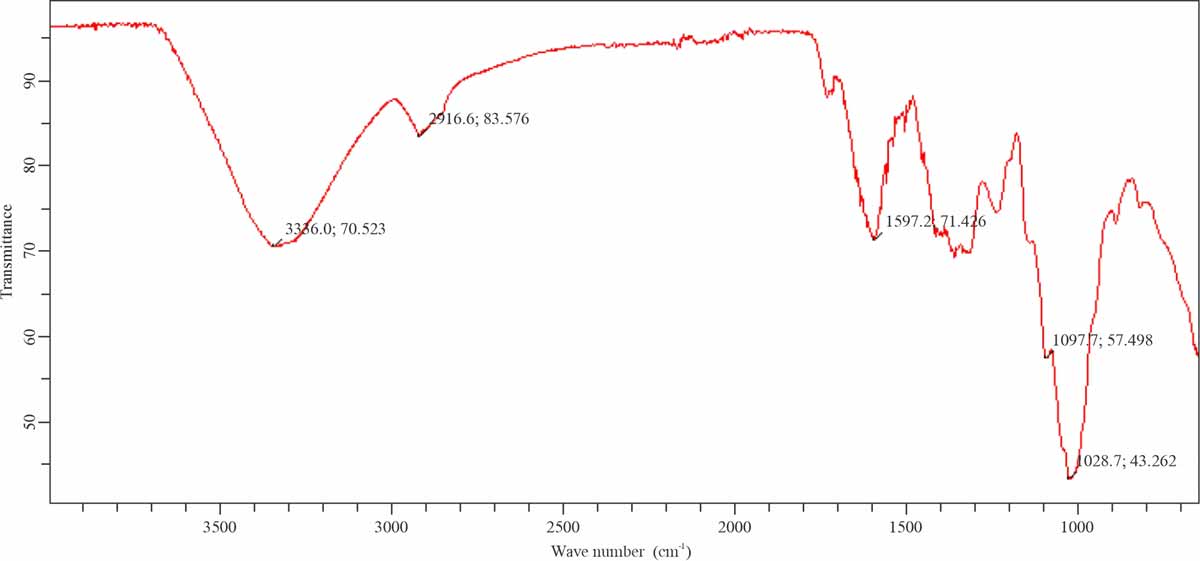
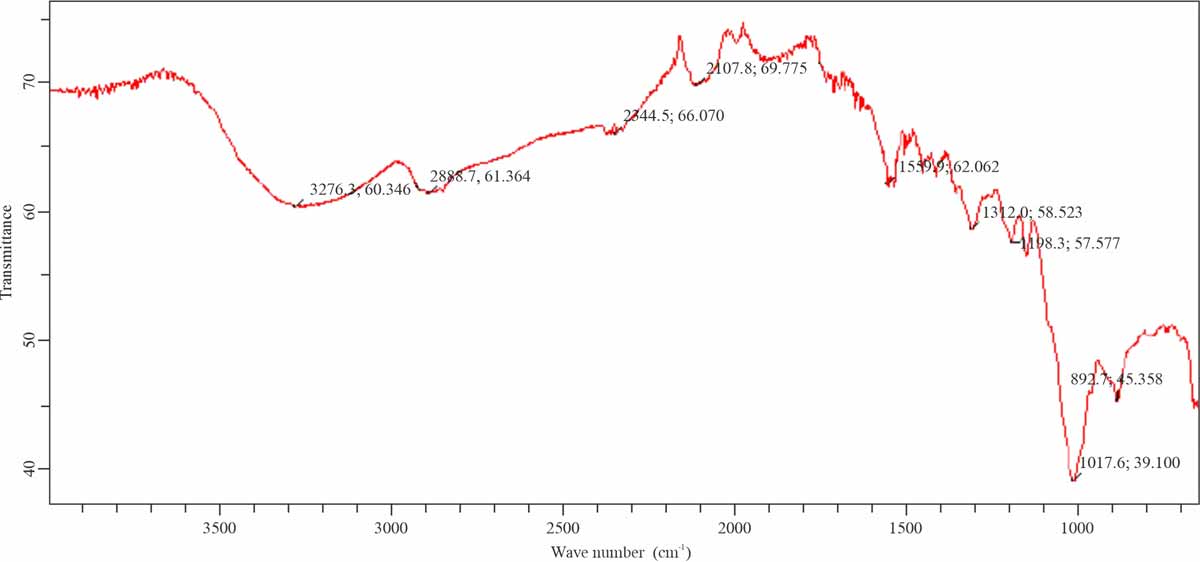
FTIR study: FTIR study identified the functional group of biosorbents surface in the range of 400-4000 cm-1. FTIR spectra of native Mustard, MUS-ZnO-PANI and MUS-ZnO-PPy were shown in Fig. 15-17. Bands appeared at 3338.0 cm-1 in native mustard might be due to the –CH bond stretching. Band intensity showed an increasing trend when treated with Polyaniline, polypyrrole and Hydrochloric acid. Moreover, a decreasing trend in band intensity appeared due to the interaction with dye molecules. Bands appeared 1563.4-1599.2 cm-1 indicated the –NH presence, which confirmed the presence of the amine group. Peaks existence in 1053.2-1034.6 cm-1 confirmed the C-O-Cvibrations. While significant observable result observed from the disappearance of bands ranging 3052.5-3415.0 cm-1 which indicate the vigorous participation of –OH and –NH2 groups in the adsorption of dye molecules on Sorbentsurface11. Thus, obtained peaks from the FTIR study indicate the presence of different active functional groups on the mustard surface. Therefore, the study proves that mustard is an economical and efficient biosorbents having the enhancing capability of anionic dye absorption.
CONCLUSION
From this study, it can be concluded that different industries produce waste water in large amount and need serious treatment to save the water. A Major hazardous compound found in textile waste water causes various diseases. Therefore, an economical technology “adsorption” utilized for the exclusion of dye from waste water. Different parameters such as pH, dose, temperature, contact time and dye concentration were optimized to enhance the elimination of dyes. Moreover, equilibrium, kinetic ad thermodynamic studies gave satisfactory results to exclude the dyes effectively.
SIGNIFICANCE STATEMENT
This study discovered and synthesized the suitable biosorbents that can be beneficial for the elimination of dyes from waste water via using the adsorption technique. Researchers can eliminate and restrain the dyes that discharged from the industries in large amount causing serious hazards for all living things. Thus, a new theory to clean and protect the environment from vulnerable substances may be arrived at.
 |
||||||||||||||||||||||||||||||||
| |
| *MUS-S considered the highest biosorbent due to its high value #while R-H considered as the lowest biosorbent due to low value |
 |
|||||||||||||||||||||||||||||
| |
| MUS-ZnO-PPy (mustard zinc oxide polypyrrole) MUS: Mustard Stem |
 |
||||||||||||||||||||||||||
| |
| |
 |
|||||||||||||||||||||||
| |
| |
 |
||||||||||||||||||||
| |
| |
 |
|||||||||||||||||
| |
| |
 |
||||||||||||||
| |
| |
 |
|||||||||||
| |
 |
|||||||||
| |
 |
|||||||
| |
 |
|||||
| |
 |
|||
| |
 |
|
| |
| MUS-ZnO-PPy (mustard zinc oxide polypyrrole) MUS: Mustard Stem |
REFERENCES
- Abourached, C., M.J. English and H. Liu, 2016. Wastewater treatment by Microbial Fuel Cell (MFC) prior irrigation water reuse. J. Cleaner Prod., 137: 144-149.
- WHO., 2019. Drinking-Water.
- Zou, H. and Y. Wang, 2017. Azo dyes waste water treatment and simultaneous electricity generation in a novel process of electrolysis cell combined with microbial fuel cell. Bioresour. Technol., 235: 167-175.
- Barwal, A. and R. Chaudhary, 2016. Feasibility study for the treatment of municipal waste water by using a hybrid bio-solar process. J. Environ. Manage., 177: 271-277.
- Park, D.H., S.J. Hwang, J.M. Oh, J.H. Yang and J.H., Choy, 2013. Polymer-inorganic supramolecular nanohybrids for red, white, green and blue applications. Progress Polymer Sci., 38: 1442-1486.
- Seid, L., D. Chouder, N. Maouche, I. Bakas and N. Barka, 2014. Removal of Cd (II) and Co (II) ions from aqueous solutions by polypyrrole particles: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng., 45: 2969-2974.
- Pandimurugan, R. and S. Thambidurai, 2016. Synthesis of seaweed-ZnO-PANI hybrid composite for adsorption of methylene blue dye. J. Environ. Chem. Eng., 4: 1332-1347.
- Asgher, M. and H.N. Bhatti, 2012. Removal of reactive blue 19 and reactive blue 49 textile dyes by citrus waste biomass from aqueous solution: Equilibrium and kinetic study. Can. J. Chem. Eng., 90: 412-419.
- Shakoor, S. and A. Nasar, 2016. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng., 66: 154-163.
- Sadaf, S. and H.N. Bhatti, 2014. Batch and fixed bed column studies for the removal of Indosol Yellow BG dye by peanut husk. J. Taiwan Inst. Chem. Eng., 45: 541-553.
- Noreen, S., H.N. Bhatti, S. Nausheen, S. Sadaf and M. Ashfaq, 2013. Batch and fixed bed adsorption study for the removal of Drimarine Black CL-B dye from aqueous solution using a lignocellulosic waste: A cost affective adsorbent. Ind. Crops Prod., 50: 568-579.
- Nausheen, S., H.N. Bhatti, S. Sadaf, Z. Farrukh and S. Noreen, 2014. Equilibrium modeling of removal of drimarine yellow HF-3GL dye from aqueous solutions by low cost agricultural waste. J. Chem. Soc. Pak., 36: 177-190.
- Subbaiah, M.V. and D.S. Kim, 2016. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms and thermodynamic studies. Ecotoxicol. Environ. Saf., 128: 109-117.
- Zhang, S.F., M.X. Yang, L.W. Qian, C. Hou, R.H. Tang, J.F. Yang and X.C. Wang, 2018. Design and preparation of a cellulose-based adsorbent modified by imidazolium ionic liquid functional groups and their studies on anionic dye adsorption. Cellulose, 25: 3557-3569.
- Noreen, S. and H.N. Bhatti, 2014. Fitting of equilibrium and kinetic data for the removal of Novacron Orange P-2R by sugarcane bagasse. J. Ind. Eng. Chem., 20: 1684-1692.
- Mahmoudabadi, T.Z., P. Talebi and M. Jalili, 2019. Removing disperse red 60 and reactive blue 19 dyes removal by using Alcea rosea root mucilage as a natural coagulant. AMB Express.
How to Cite this paper?
APA-7 Style
Saher,
S., Noreen,
S., Nazish,
Z. (2021). Removal of Industrial Dye via Biohybrid Composites Characterized by FTIR and SEM. Asian Journal of Emerging Research, 3(2), 137-140. https://doi.org/10.3923/ajerpk.2021.137.140
ACS Style
Saher,
S.; Noreen,
S.; Nazish,
Z. Removal of Industrial Dye via Biohybrid Composites Characterized by FTIR and SEM. Asian J. Emerg. Res 2021, 3, 137-140. https://doi.org/10.3923/ajerpk.2021.137.140
AMA Style
Saher
S, Noreen
S, Nazish
Z. Removal of Industrial Dye via Biohybrid Composites Characterized by FTIR and SEM. Asian Journal of Emerging Research. 2021; 3(2): 137-140. https://doi.org/10.3923/ajerpk.2021.137.140
Chicago/Turabian Style
Saher, Sabeen, Saima Noreen, and Zunaira Nazish.
2021. "Removal of Industrial Dye via Biohybrid Composites Characterized by FTIR and SEM" Asian Journal of Emerging Research 3, no. 2: 137-140. https://doi.org/10.3923/ajerpk.2021.137.140

This work is licensed under a Creative Commons Attribution 4.0 International License.



