Brain Serotonin-2C Receptor Regulation in Schizophrenia Rat Model
| Received 26 May, 2019 |
Accepted 23 Jul, 2019 |
Published 15 Aug, 2019 |
Background and Objective: Schizophrenia is a serious psychiatric disorder which affects about 1% of the entire population. Hallucinations and delusions in schizophrenia are characterized as the positive symptoms of the disorder whereas social withdrawal, lack of motivation and poverty of speech are classified as negative symptoms. The over activity of mesolimbic dopamine neurotransmission is involved in schizophrenia. Serotonin acting via 5HT2C receptors inhibits dopamine neurotransmission. The objective of the present study was to investigate the 5-HT2C receptor responsiveness in apomorphine induced-behavioral schizophrenic like symptoms. Materials and Methods: The animals treated with apomorphine (a dopamine D1 & D2 receptor agonist) for two weeks were given challenge doses of mCPP (5-HT2C agonist). Results: Behavioral effects of mCPP showed that it attenuate apomorphine-induced sensitization as 5-HT2C receptors have inhibitory control over dopamine neurons. Conclusion: It is concluded that both the doses of mCPP (0.25mg/kg & 0.5mg/kg) significantly reduced motor activity in apomorphine induced hyperactive rats.
INTRODUCTION
Schizophrenia, also referred as psychosis, is a serious and debilitating psychiatric disorder involving generalized cognitive impairment specified by hallucinations and delusions. It also produces other deficits such as deficits in perception, memory, executive functioning and attention1. The onset of the disease is generally between ages of 15-40 years2. It was estimated that about 1% of the world’s population is suffering from schizophrenia3. According to the dopamine hypothesis of schizophrenia4overstimulation of dopamine receptors5 (particularly D2) are responsible for causing hyperactivity in dopamine neurotransmission and thus producing the positive symptoms of the disease. Psychotic symptoms include hallucinations and delusions. Deficit symptoms such as alogia (poverty of speech), anhedonia (lack of pleasure seeking behavior), affective flattening, social withdrawal, mood swings etc. are generally characterized as negative symptoms of the disease. Antipsychotics are highly effective in treating positive symptoms of schizophrenia. However, as a negative aspect, they produce extrapyramidal symptoms (EPS) when given in doses within the clinically effective range6. The extra pyramidal side effects of antipsychotics comprise a range of postural and motor problems that include akathesia (motor restlessness), dystonia (abnormal body tone) and Parkinsonism (rigidity, tremor, stooped posture etc.). Doses of 1.0 mg/kg of apomorphine when administered repeatedly produce sensitization in rats which can be evaluated in an open field7,8. Within the brain, 5-HT2C receptors are found to control dopamine function in the mesolimbic pathway, having inhibitory effect on dopamine neurotransmission9,10. m-chlorophenylpiperazine (mCPP) has a major role in the regulation of serotonergic neurons11.The mechanism of action of mCPP involves direct stimulation of postsynaptic 5-HT receptors12 specifically of 5-HT2C receptors13 . The findings from the current research may provide a gateway for developing dopamine and serotonin dual acting agents for the treatment of schizophrenia and would also help in improving the existing treatment strategies for this disabling disease.
The present study was designed to monitor serotonin 5HT2C receptor responsiveness in apomorphine-induced schizophrenia like symptoms. The animals treated with apomorphine and exhibiting sensitization would be given challenge doses of mCPP to get an understanding of regulation of 5HT2C receptor function in schizophrenia.
MATERIALS AND METHODS
Experimental Animals: Animal handling was done according to the guidelines provided by ‘Guide for the care and use of laboratory animals’, The National Academies Press, Washington D.C, USA and the Institutional Animal Ethics Committee (IAEC), Animal Study Protocol no. 2016-0002. Albino-Wistar rats having weight of about 180-220 gm were taken from the animal house, ICCBS, University of Karachi. They were housed individually and kept under 12 h light dark cycle and controlled room temperature (24±2°C) with free access to standard diet and water. Rats were acclimatized to different handling methods to avoid psychological stress and interference of the environment. The total time duration of the study was about 6 months. All experiments were performed in Animal House core facility laboratory, ICCBS, University of Karachi.
Drugs: Both drugs apomorphine–HCl and m-chlorophenylpiperazine-HCl (Sigma, St Louis, Missouri, USA) were dissolved in saline and injected subcutaneously to animals. Drug solutions were prepared daily before injection. Control animals were injected with saline (1.0 ml/kg).
Experiment No. 1:
Dose related effects of apomorphine on motor behavior in rats: Twenty four animals were randomly divided into 4 groups (n=6). Three groups were injected with apomorphine (0.25mg/kg, 0.5mg/kg and 1mg/kg) and 1 group was injected with saline. Drug and saline were injected subcutaneously in rats for two weeks to select a dose that produces hyperactivity and induces sensitization in rats. Animals were familiarized in activity boxes for 30 minutes and then activity was monitored for 10 minutes. Open field activity was monitored 40 minutes post injection for 5 minutes.
Experiment No. 2:
Behavioral effects of mCPP in rats pretreated with apomorphine for two weeks: Twenty four animals were equally divided into two groups (n=12), (a) saline and (b) 1mg/kg apomorphine. Saline or drug was injected subcutaneously in animals for two weeks. mCPP treatment was started on day 15. 2 doses of mCPP (0.25 & 0.5mg/kg) were given subcutaneously to rats for three days. Animals were then sub-divided into 6 groups; (a) saline + saline (mCPP 0mg/kg), (b) saline + mCPP 0.25mg/kg (c) saline + mCPP 0.5mg/kg (d) apomorphine+ saline (mCPP 0mg/kg) (e) apomorphine + mCPP 0.25mg/kg (f) apomorphine + mCPP 0.5mg/kg. Home cage activity and open field activity was monitored on day 15. Acquisition and retention of memory was monitored on day 15 and day 16.
Behavioral Analysis
Home Cage Activity: It was performed in Skinners box which a square is shaped transparent activity box made of dimensions 26×26×26 cm3. Rats were placed in the cage 30 minutes after administration of the drug for familiarization with the environment. Number of cages crossed was then noted for 10 minutes to analyze motor activity in rats after drug administration.
Open Field Activity: The open field is an open arena covering square area of 76 × 76 cm having opaque walls of 42 cm height. The floor is divided into 25 equal squares. The activity is done in a noiseless room under white light to avoid any unwanted effect. Animals are first kept in the center square of the open field. For a cut off time of five minutes latency to move and number of squares crossed by the animal is monitored.
Statistical Analysis: Results are represented as Mean ± Standard Deviation (SD). Analysis was performed by SPSS Software version 15. Two-way ANOVA and repeated measures design was performed. Posthoc analysis was done via Tukey’s Analysis and p values ≤ 0.05 were considered as significant.
RESULTS
Dose related effects of apomorphine on activity in a familiar environment: It is shown in Figure 1 that dose related effects of repeated administration of apomorphine at doses of 0.25mg/kg, 0.5mg/kg and 1 mg/kg on activity of animals in a familiar environment. The activity was monitored daily for 10 minutes. Two-way ANOVA repeated measures design revealed significant effect of apomorphine (F=26.697, df=13,260 p<0.01), repeated measures (F=421.614, df=3, 20 p<0.01) and a significant (F=20.074, df=39,260 p< 0.01) interaction between apomorphine and repeated measures. Post hoc test showed that single and repeated administration of apomorphine at dose of 1mg/kg significantly increased motor activity of rats in the activity box every day. These results suggested that behavioral sensitization was produced after repeated administration of apomorphine at dose 1mg/kg but not at lower doses.
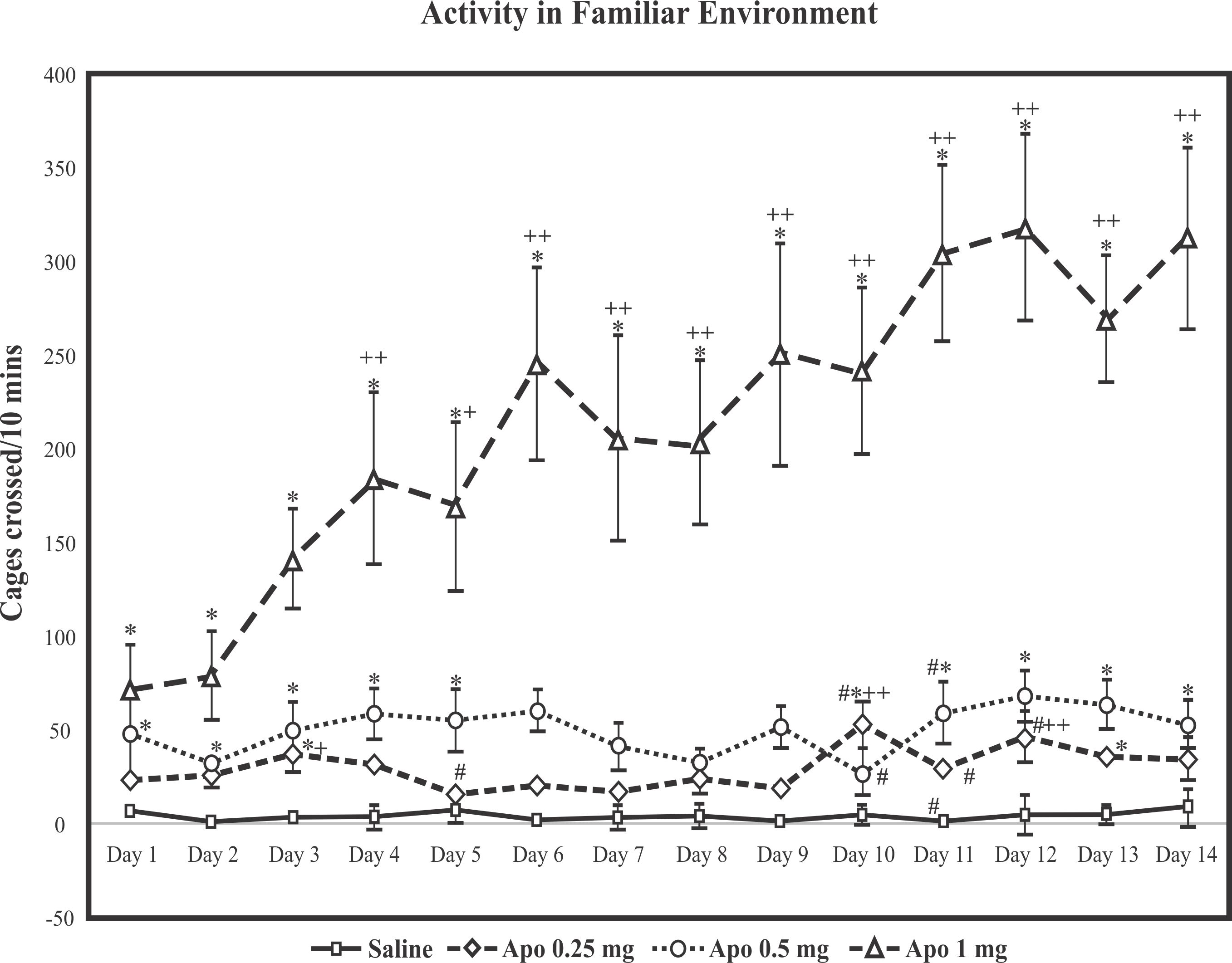 |
Fig 1: Values are means ±SD. Significant difference by Tukey’s test: * p<0.01 from same day saline injected animals,+ p<0.05, ++p<0.01 from day 1 values of similarly treated animals and # from preceding day values of similarly treated animals following Two-way ANOVA |
Dose related effects of apomorphine in novel environment of an open field: Figure 2 showed that dose related effects of repeated administration of apomorphine at doses of 0.25mg/kg, 0.5mg/kg and 1 mg/kg on activity in a novel environment of an open field. Two-way ANOVA repeated measures design showed significant effects of apomorphine (F=170.65, df=3,20 p<0.01), repeated measures (F=180.351 df=2,40) and a significant interaction between repeated measures and apomorphine (F=302.291, df=3,20 p<0.01). Post hoc analysis showed a significant increase in exploratory activity of rats treated with the highest dose of apomorphine (1mg/kg). Whereas upon single administration of apomorphine it was observed that the activity of all doses of apomorphine decreased in novel environment. At day 6 and day 10 the exploratory activity significantly increased in animals treated with 0.5mg/kg and 1mg/kg of apomorphine but the activity was found to be very high in animals treated with 1mg/kg apomorphine suggesting behavioral sensitization in the respective group.
 |
Fig 2: Values are means ±SD. Significant difference by Tukey’s test * p<0.01 from same day saline injected animals and + p<0.01; from day 1 value of similarly treated animals following Two-way ANOVA |
mCPP induced hypolocomotry effects in apomorphine pre-treated rats: Figure 3a is showing the effects of mCPP (0 mg/kg, 0.25mg/kg and 0.5mg/kg) in apomorphine pre-treated rats on activity in a familiar environment. The test was performed in skinners box after 1 hour of administration of mCPP and after 30 minutes of familiarization, the activity was performed for 10 minutes. Two-way ANOVA revealed significant effect of mCPP (F=17.588, df=2, 18 p<0.01) and the effect of apomorphine (F=38.40, df=1, 18 p<0.01) but the effect of interaction between apomorphine x mCPP (F=0.713, df=2, 18 p >0.05) was not significant. Post hoc analysis showed that at 0 mg/kg of mCPP the motor activity of rats was higher in rats pre-treated with apomorphine showing sensitization. At low dose of mCPP the motor activity of rats was significantly reduced in apomorphine pre-treated rats indicating that mCPP produced motor deficits in the respective group. At higher dose of mCPP i.e 0.5mg/kg the rats showed further decrease in motor activity which was found to be significant in both groups i.e the apomorphine pre- treated and the saline treated rats.
In Figure 3b effects of mCPP (0 mg/kg, 0.25mg/kg and 0.5mg/kg) in apomorphine pre-treated rats showed on activity in a novel environment. The test was performed in an open field after 1 day of administration of mCPP for 5 minutes. Two-way ANOVA revealed significant effect of apomorphine (F=44.053, df=1, 18 p <0.01) and mCPP (F=16.994, df=2,18 p<0.01) but the interaction between apomorphine x mCPP (F=0.027, df=2,18 p>0.05) was not significant. Post hoc analysis revealed that at 0mg/kg of mCPP the motor activity was high in apomorphine pre-treated rats but it was significantly reduced in rats treated with mCPP at low dose (0.25mg/kg). The rats previously treated with saline also showed a slight reduction in activity however the difference was not significant. At high dose of mCPP (0.5mg/kg) the activity was further decreased in both the groups; the saline treated and apomorphine pre-treated suggesting that mCPP produced motor deficits and hypoactivity in rats.
DISCUSSION
Repeated administration of apomorphine produced sensitization and 5-HT2C agonist-mCPP attenuated it and produced hypolocomotion in rats. The Dopamine system plays an important role in psychostimulant-induced hyperactivity and addiction14.This increase in activity, also known as sensitization, is caused by changes in the mesolimbic and mesostriatal dopamine pathways15. Sensitization is also described as drug wanting effect and the behavior is often used as rat model for schizophrenia16 . Psychostimulant drugs like amphetamine, cocaine and apomorphine also considered as drug of abuse. Amphetamine induces sensitization and increases dopamine neurotransmission by acting presynapticaly, whereas apomorphine directly stimulates DA receptors15. In the present study apomorphine is selected as a psychostimulant to produce behavioral sensitization which is used as a rat model of schizophrenia. The first experiment, the dose response study was designed to select an optimum dose to be proceeded for further experiments. Three doses (0.25mg/kg, 0.5mg/kg & 1 mg/kg) were selected after literature review17. According to previous studies done on apomorphine, its plasma peak occurs between 15 and 20 minutes after injecting the drug so animals were tested 30 minutes post injection for familiar environment and 40 minutes post injection for novel environment8. The sensitization developed after repeated administration of apomorphine (1.0 mg/kg) can be monitored in an open field7. Apomorphine at doses 1mg/kg significantly increased motor activity of rats. There was also a significant increase in exploratory activity of rats treated with the highest dose of apomorphine (1mg/kg) as monitored in novel environment of an open field.5-HT2C receptors are extensively found in the regions of the brain that are found to link with the neuropathophysiology of schizophrenia, particularly the cerebral cortex and limbic structures18.
They are located in the cell bodies of dopaminergic neurons of the nigrostriatal and mesolimbic pathway19 . Evidence suggested that 5-HT2C receptor agonists decrease mesocorticolimbic dopamine transmission selectively which may be of therapeutic importance in psychiatric disorders like schizophrenia20.
To study effects of mCPP in hyperactive rats, pre-treatment with the selected dose of apomorphine (1mg/kg) repeatedly for two weeks was used to create sensitization and then challenge doses of mCPP was given to monitor induced hypoactivity to get an insight into the regulation of 5HT2C receptor function in schizophrenia rat model. The activity significantly reduced in rats treated with mCPP at both low dose (0.25mg/kg) and high dose (0.5mg/kg). The rats previously treated with saline also showed a slight reduction in activity with mCPP treatment. Similar effects of mCPP were observed in novel environment of open field. This suggested that mCPP produces motor deficits in rat models of schizophrenia and attenuated apomorphine-induced hyperactivity.
CONCLUSION
mCPP, the 5-HT2C agonist attenuated apomorphine-induced sensitization as 5-HT2C receptors have inhibitory control over dopamine neurons. Therefore, it may be concluded that over expression of 5-HT2C receptors is involved in producing parkinsonian like effects in patients treated with antipsychotics and 5-HT2C receptor antagonist may found to be beneficial in reducing parkinsonian like symptoms in these patients.
SIGNIFICANCE STATEMENT
This study discovered that over expression of 5-HT2C receptors is involved in producing Parkinsonian like effects in patients. And 5-HT2C receptor antagonist can be beneficial in reducing the Parkinsonian like symptoms that will help the researchers to uncover the critical areas of this disease that many researchers were not able to explore.
REFERENCES
- Green, M.F., W.P. Horan and J. Lee, 2015. Social cognition in schizophrenia. Nature Rev.: Neuroscience, 16: 620-631.
- Akhtar, S., 2010. Schizophrenia in Pakistan. The International Society of Psychological and Social Approaches to Psychosis.
- McGrath, J., S. Saha, D. Chant and J. Welham, 2008. Schizophrenia: A concise overview of incidence, prevalence and mortality. Epidemiol. Rev., 30: 67-76.
- Van Rossum, J.M., 1966. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Archiv. Int. Pharmacodynam. Ther., 160: 492-494.
- Laruelle, M., L.S. Kegeles and A. Abi-Dargham, 2003. Glutamate, dopamine and schizophrenia. Ann. NY Acad. Sci., 1003: 138-158.
- Haleem, D., 2015. 5-HT1A receptor-dependent control of nigrostriatal dopamine neurotransmission in the pharmacotherapy of Parkinson's disease and schizophrenia. Behav Pharmacol. 26: 45-58.
- Ikram, H., S. Ahmad and D.J. Haleem, 2011. Effects of apomorphine on locomotive activity and monoamine metabolism: A dose related study. Pak J Pharm Sci. 24: 315-321.
- Braga, P.Q., J.P. Galvanho, E. Bloise, R.J. Carey and M.P. Carrera, 2009. The expression of locomotor sensitization to apomorphine is dependent on time interval between injection and testing. Pharmacol. Biochem. Behav., 91: 278-282.
- Bubar, M.J. and K.A. Cunningham, 2007. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience, 146: 286-297.
- Di Giovanni, G., V. Di Matteo, M. Di Mascio and E. Esposito, 2000. Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: A combined in vivo electrophysiological and microdialysis study. Synapse, 35: 53-61.
- Murphy, D.L., K.P. Lesch, C.S. Aulakh and T.A. Pigott, 1991. Serotonin-selective arylpiperazines with neuroendocrine, behavioral, temperature and cardiovascular effects in humans. Pharmacol. Rev., 43: 527-552.
- Baumann, M.H., D.C. Mash and J.K. Staley, 1995. The serotonin agonist m-chlorophenylpiperazine (mCPP) binds to serotonin transporter sites in human brain. Neuroreport, 6: 2150-2152.
- Bilkei-Gorzo, A., I. Gyertyan and G. Levay, 1998. mCPP-induced anxiety in the light-dark box in rats-A new method for screening anxiolytic activity. Psychopharmacology, 136: 291-298.
- Robinson, T.E. and K.C. Berridge, 2000. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction, 95: 91-117.
- Battisti, J.J., C.B. Shreffler, N.J. Uretsky and L.J. Wallace, 2000. NMDA antagonists block expression of sensitization of amphetamine- and apomorphine-induced stereotypy. Pharmacol. Biochem. Behav., 67: 241-246.
- Stewart, J. and A. Badiani, 1993. Tolerance and sensitization to the behavioral effects of drugs. Behav. Pharmacol., 4: 289-312.
- Deleu, D., Y. Hanssens and M.G. Northway, 2004. Subcutaneous apomorphine: An evidence-based review of its use in Parkinson's disease. Drugs Aging, 21: 687-709.
- Abramowski, D., M. Rigo, D. Duc, D. Hoyer and M. Staufenbiel, 1995. Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology, 34: 1635-1645.
- Pompeiano, M., J.M. Palacios and G. Mengod, 1994. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol. Brain Res., 23: 163-178.
- Kahn, R.S. and S. Wetzler, 1991. m-Chlorophenylpiperazine as a probe of serotonin function. Biol. Psychiat., 30: 1139-1166.
How to Cite this paper?
APA-7 Style
Zafar,
M., Afroz,
R., Nawaz,
S., Salman,
T., Haleem,
D.J. (2019). Brain Serotonin-2C Receptor Regulation in Schizophrenia Rat Model. Asian Journal of Emerging Research, 1(2), 91-97. https://doi.org/10.3923/AJERPK.2019.91.97
ACS Style
Zafar,
M.; Afroz,
R.; Nawaz,
S.; Salman,
T.; Haleem,
D.J. Brain Serotonin-2C Receptor Regulation in Schizophrenia Rat Model. Asian J. Emerg. Res 2019, 1, 91-97. https://doi.org/10.3923/AJERPK.2019.91.97
AMA Style
Zafar
M, Afroz
R, Nawaz
S, Salman
T, Haleem
DJ. Brain Serotonin-2C Receptor Regulation in Schizophrenia Rat Model. Asian Journal of Emerging Research. 2019; 1(2): 91-97. https://doi.org/10.3923/AJERPK.2019.91.97
Chicago/Turabian Style
Zafar, Munnum, Rushda Afroz, Shazia Nawaz, Tabinda Salman, and Darakhshan J. Haleem.
2019. "Brain Serotonin-2C Receptor Regulation in Schizophrenia Rat Model" Asian Journal of Emerging Research 1, no. 2: 91-97. https://doi.org/10.3923/AJERPK.2019.91.97

This work is licensed under a Creative Commons Attribution 4.0 International License.




