Effect of Arsenic on Growth and Cell Division in Root Tip Cells of (Allium sativum L.)
| Received 26 Feb, 2021 |
Accepted 26 Apr, 2021 |
Published 15 Jun, 2021 |
Background and Objectives: Arsenic trioxide is a toxic element and effect on human health and as well as plants and bacteria. The effect of genotoxicity and cytotoxicity of Arsenic trioxide was investigated using both morphological and chromosomal assays. The present study was designed to examine the effect of Arsenic trioxide (As2O3) on germination, growth and cell divisions. Materials and Methods: The Allium sativum L. bulbs were exposed to different concentrations of Arsenic trioxide in different time interval and mean root length of garlic were measured and study chromosome abnormality. Results: The root meristems of germinated Allium sativum L. to reveal the cytogenic effect and chromosomal abnormalities induced by Arsenic trioxide. There was significant decrease in root length and number as concentration increased in the experiment and mitotic index decreased as concentration of arsenic trioxide increased. Conclusion: Total chromosomal abnormality increased significantly as concentration of Arsenic trioxide increased.
INTRODUCTION
Heavy metal pollution is one of the serious environmental problems all over the world. Heavy metal pollution are usually generated through many industrial processes. Various industries such as metals finishing industries, metallurgy, tannery, battery manufacturing industries, glass factories, plastic industries etc. release heavy metals as a by products and then contaminates the soil1. Heavy metals are carcinogenic for human and their genotoxicity effect of heavy metals like chromium, cadmium, nickel, iron and lead have been reported in various biological systems. Toxic effect of many heavy metals including arsenic, cadmium, lead, mercury, chromium, nickel, manganese and iron, is a widespread problem in India and other developing countries. Excessive heavy metal stress causes oxidative damage. Arsenic is unique among the heavy metals because it naturally occurs in several oxidation states. Toxic effect of arsenic (As) in biota have been widely reported.
Arsenic (As) is non-essential but toxic for plants. Plants do not possess specific mechanisms for As uptake and transport. Arsenic which most likely enters the plant cell through the existing mineral uptake machinery and also cause effect on human health. It is well documented that As exposure in soil and cause growth inhibition related to reduction of mitotic activity, induction of chromosome disorders and nuclear abnormalities in the roots apical meristems of Allium sativum L2. But genotoxicity effect of Arsenic has not been fully evaluated in case of Allium sativum L. Allium sativum L. is well known and commonly used in many laboratories because it is a useful biomarker for environmental monitoring. Allium sativum L. use has many advantages such as low cost, a large number of roots, short testing time, large cells with excellent chromosome conditions, and easiness of observation of abnormal phenomena on chromosomes during mitosis3. Limited information is available on the effects of As on root growth, cell division and chromosome abnormality in root tip cells of Allium sativum L. Our objective was to study the effects of Arsenic(As) on root growth, cell division and chromosome abnormalities in root tips of Allium sativum L. The toxicity of As compounds is most probably based on an oxidative DNA impairment. Optimal concentrate of heavy metals affects the agronomic trait of plants. Cytogenetic test analyses the frequency and type of chromosome aberrations in mitotic cells of Allium sativum L. The data obtained here from this experiment will be very useful to better understand the mechanisms of As induced cell toxicity.
MATERIALS AND METHODS
In vivo Culture and treatment of Arsenic on Allium sativum L. plantLaboratory experiments: Arsenic trioxide (As2O3) salt was used as arsenic source for the present study 500ml stock solution of Arsenic trioxide was prepareand from stock solution the different concentrations (20, 50, 100 ppm) of arsenic trioxide solution were prepared and used for the germination and growth rate studies4.
Soil and pot preparation: Soil was collected from garden. Soil sample was placed in 4 pots for planting and different concentration of arsenic trioxide solution (20, 50, and 100 ppm) were added (20ml) in 3 different pot containing soil except the pot marked as control. Healthy garlic blubs were grown in different potsfor study of germination rate and growth rate.
Determination of plant growth and germination rate: Germination rate was determined after 10 days. Total plant height, leaf size, root length, stem length were measured after 10, 20, 30, 40 days respectively using meter scale.
Cytological studies: The root tips were taken after 7 days and used for cytological studies. The root tips were collected between 9 am to 9.45 am from different concentrations of arsenic treated Allium sativum L. for cytological investigation. Root tip of Allium sativum L. squashes were made by using iron alum, haematoxyl in squash technique5. This technique was found to be suitable for the cytological investigation and observed under microscope for chromosomal abnormalities. The number of cells at division phase, abnormal cells and chromosomal aberrations were noted in each concentrations and mitotic index was calculated6.
Mitotic index= (Number of dividing cells/Total number of cells) x 100
RESULTS AND DISCUSSION
Arsenic pollution is a signifcant environmental Health problem that affects many physiological and biochemical processes. The most common effects of arsenic on plants are stunted growth, leaf chlorosis and on many key enzymes in various metabolic pathways7. In this study reductions root length of Allium sativum L. were observed with increasing arsenic concentrations. The present study indicated that As presented mutagenic activity on Allium sativum L. In the present study Allium sativum L. blubs were germinated in control as well as different Arsenic trioxide concentrations.
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
|
|||||||
| Sr. No |
Heavy metal Concentration(ppm) As2O3 |
Number of seeds | Day | Number of seed germinate | Germination rate (%) | ||
| 1 | Control | 2 | 10 | 2 | 100 | ||
| 2 | 20 | 2 | 10 | 2 | 100 | ||
| 3 | 50 | 2 | 10 | 2 | 100 | ||
| 4 | 100 | 2 | 10 | 2 | 100 | ||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
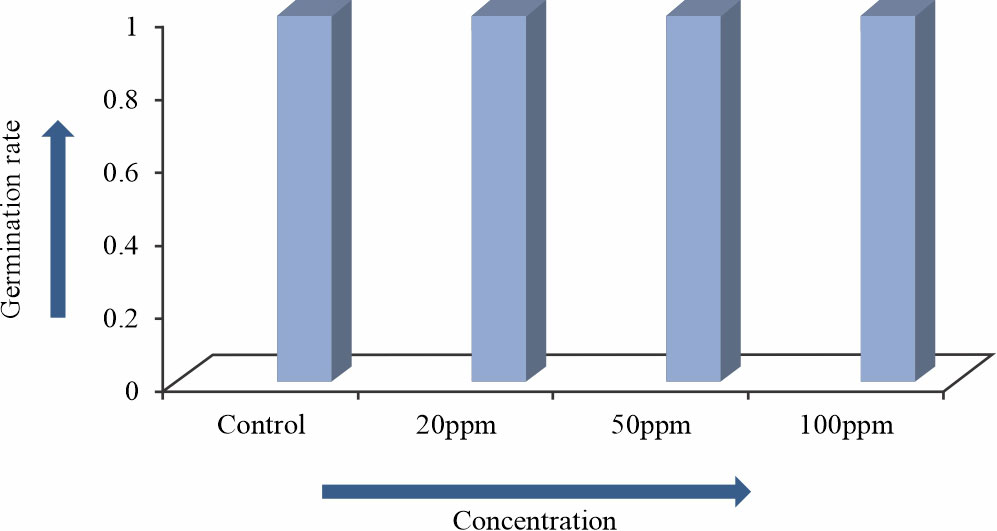
Arsenic trioxide not inhibited the germination of Allium sativum L. (Fig. 1,2, Table 1, Fig. 4) up to 100ppm concentration but it was found that germination rate of some plant like Oryza sativa inhibited by 100ppm concentration of Arsenic trioxide8. The growth of Allium sativum L. were gradually decreased with the increasing Arsenic trioxide concentrations. In 20, 50 and 100 ppm condition height of Allium sativum L. was recorded at 22cm ,18cm and 14cm respectively and in control condition Allium sativum L. height was 24cm because Arsenic trioxide inhibited the growth (Fig. 3, Table 2).
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
|
|||||||
| Sr. No | Heavy metal concentration (ppm) | Number of root | Root length (cm) | Day | Height (cm) | ||
| 1 | Control | 27 | 5 | 40 | 24 | ||
| 2 | 20 | 20 | 4 | 40 | 22 | ||
| 3 | 50 | 18 | 3.5 | 40 | 8 | ||
| 4 | 100 | 14 | 3 | 40 | 14 | ||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
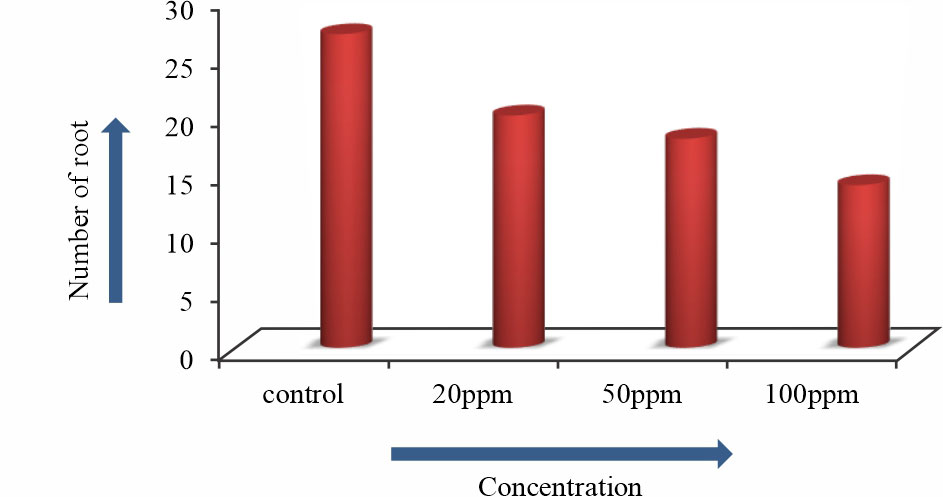
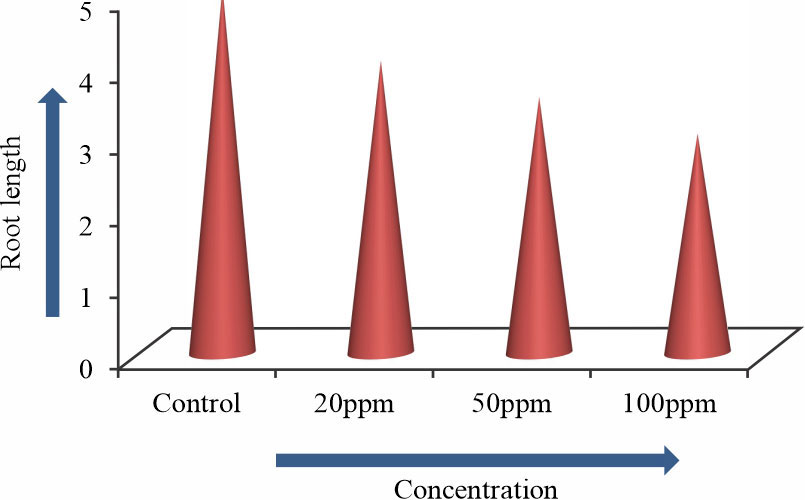
The number of roots and root length of Allium sativum L. were gradually decreased with the increasing Arsenic trioxide concentrations. In 100ppm arsenic concentration root length of Allium sativum L. was decreased (Fig. 5, Table 2, Fig. 6, 7). To study the effect of Arsenic trioxide on meristematic cells of Allium sativum L. roots were analyzed to record as mitotic index9. Germinated roots, which were exposed to control condition did not show significant mitotic inhibition.
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
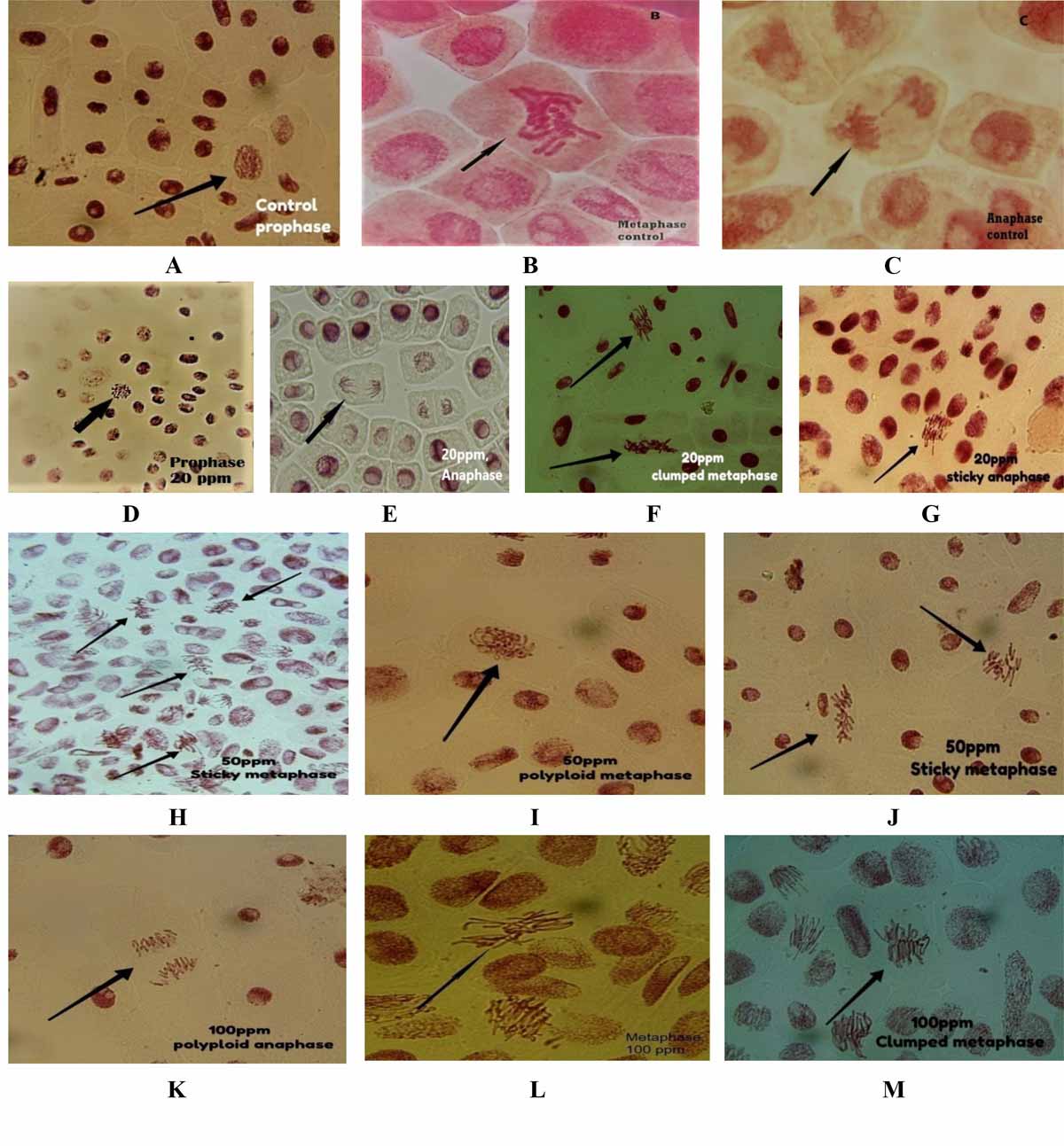
The cytotoxicity and genotoxicity of Arsenic trioxide was investigated by observing the mitotic index. Additionally, the primary action of Arsenic trioxide on the mitotic spindle promoted spindle related abnormalities such aslaggard chromosome and bridges during cell division (Fig. 8)10. Heavy metal induced genotoxic effects on plants and other biological systems depend on the oxidation state of metal, its concentration and duration of the exposure. Effects of arsenic more pronounced at higher concentration and at longer duration of exposure. Concerning cyto/genotoxic potency of Arsenic trioxide showed a strong phytotoxic effect, affecting Allium sativum L. at the concentration of 100ppm cause lethal effect on chromosome. Reduction in cell division due to As2O3 is well documented effect in many plants. The result of this investigation showed a significant reductionof mitotic index in Allium sativum L. Root meristematic cells within the presence of arsenic trioxide which might be due to inhibition of mitosis or extension of cell cycles11. This may attribute to the blocking of cell division by Arsenic trioxide at the end of the prophase. In this case, this metal may be considered as pre metaphase inhibitor. Arsenic induced mitotic chromosome bridges, chromosome stickiness and micronuclei formation which are biomarkers of genotoxic events and chromosomal instability (Table 3, Fig. 8)12. Chromosome bridges involving in one or more chromosomes were found at anaphase in the As treated groups. Chromosome bridges originated in cell from dicentric chromosomes due to the misrepair of DNA breaks13. Also chromosome bridges occur due to telomere end fusions, and when separation of sister chromatids was defective at anaphase due to the failure of decatenation14. Sticky chromosome indicate highly toxic effects of arsenic in cell division, probably leading to cell death (Fig. 8 G, H). Cells with chromosome stickiness significantly increased with increasing As concentrations (Table 3, Fig. 8).
|
||||||||
| Concentration | No . ofdividing cells | Laggard | Vagrant | Sticky Chromosome | Chromosomal bridge | Total aberration Ratio % | ||
| Control | 209 | 2 | 1 | 0 | 0 | 1.43 | ||
| 20ppm | 172 | 0 | 0 | 2 | 1 | 1.71 | ||
| 50ppm | 167 | 1 | 0 | 2 | 0 | 2.43 | ||
| 100ppm | 152 | 0 | 1 | 0 | 3 | 2.22 | ||
|
||||
| Concentration | No. ofdividing cells | Mitotic index % | ||
| Control | 209 | 20.9 | ||
| 20 ppm | 172 | 17.2 | ||
| 50 ppm | 167 | 16.7 | ||
| 100 ppm | 152 | 15.2 | ||
The mitotic index of chromosomes decreased with increasing As concentrations and duration of treatment. The mitotic index decreased significantly 20 ppm - 100 ppm. As during the whole treatment when compared with the control (Table 4). Chromosomal aberrations were induced at all the tested concentration. The most frequent aberration were bridges and sticky chromosome (Table 3). The frequency of cell division in root tips significantly increased at low concentrations of As and significantly decreased prolonging the duration of treatment at higher concentrations of As (Table 4). The inhibition of root growth resulted from inhibiting the cell division of root tips of Allium sativum L. In Allium sativum L. Sticky chromosome represent chromosome abnormality with sticky surface and probably lead to cell death in root tips15. Sticky chromosome at metaphase and anaphase stage were abundant in the Allium test at high concentration indicating its toxicity16. It was also found that significant increase of the frequency of abnormal cells with chromosomal aberration in Allium sativum L.root meristematic cell with the addition ofarsenic trioxide (As2O3).
CONCLUSION
Arsenic trioxide was known for its toxicity. Arsenic trioxideeffects on plants, animals, chromosomes, genes etc. In plants Arsenic trioxide adversely affects the growth and yield of the plant. In this study Arsenic played a negative role as a growth inhibitor. Therefore, cultivation of garlic (Allium sativum L.) on the area where Arsenic trioxide contaminated soil is being helpful for irrigation. The present study provides additional and valuable information about the toxic effects of arsenic trioxide by evaluating genetic and cytological end point on Allium sativum L. in different concentrations of arsenic trioxide. This study showed the necessity of combining physico-chemico analysis with cytogenetic approaches to better understand the toxicity of Arsenic trioxide. However, a chain of in-vitro study is required to know the biochemistry of the entire phenomena and to reveal the mechanism behind the positive and toxic effect of Arsenic on the garlic plant. This could open up a new area of research.
ACKNOWLEDGEMENT
The authors acknowledged the PG Department of Botany, Ramananda College, Bishnupur, Bankura for providing financial assistance to carry out this research work.
SIGNIFICANCE STATEMENT
Arsenic trioxide is a very toxic heavy metal and it has very significant effects on Allium sativum L. It has been observed that Arsenic trioxide effects on root length, growth and chromosome structure in this study. Further it was also revealed that Chromosome abnormality increased significantly as concentration of arsenic trioxide increased and Mitotic index decreased as concentration of arsenic trioxide increased.
REFERENCES
- Andrioli, N.B., S. Soloneski, M.L. Larramendy and M.D. Mudry, 2012. Cytogenetic and microtubule array effects of the zineb-containing commercial fungicide formulation azzurro® on meristematic root cells of Allium cepa L. Mutat. Res./Genet. Toxicol. Environ. Mutagen., 742: 48-53.
- Özkara, A., D. Aky?l, S.F. Erdo?mu? and M. Konuk, 2011. Evaluation of germination, root growth and cytological effects of wastewater of sugar factory (afyonkarahisar) using Hordeum vulgare bioassays. Environ. Monit. Assess., 183: 517-524.
- Přibyl, P., V. Cepák and V. Zachleder, 2005. Cytoskeletal alterations in interphase cells of the green alga Spirogyra decimina in response to heavy metals exposure: I. the effect of cadmium. Protoplasma, 226: 231-240.
- Cao, F., F. Chen, H. Sun, G. Zhang, Z.H. Chen and F. Wu, 2014. Genome-wide transcriptome and functional analysis of two contrasting genotypes reveals key genes for cadmium tolerance in barley. BMC Genomics.
- Ateeq, B., M. Abul Farah, M.N. Ali and W. Ahmad, 2002. Clastogenicity of pentachlorophenol, 2,4-D and butachlor evaluated by Allium root tip test. Mutat. Res./Genet. Toxicol. Environ. Mutagen., 514: 105-113.
- Gori, R., F. Ferrini, F.P. Nicese and C. Lubello, 2000. Effect of reclaimed wastewater on the growth and nutrient content of three landscape shrubs. J. Environ. Hortic., 18: 108-114.
- Abedin, M.J. and A.A. Meharg, 2002. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil, 243: 57-66.
- Chakraborty, R., A.K. Mukherjee and A. Mukherjee, 2008. Evaluation of genotoxicity of coal fly ash in Allium cepa L. root cells by combining comet assay with the allium test. Environ. Monit. Assess., 153: 351-357.
- Chauhan, L.K., P.N. Saxena and S.K. Gupta, 2001. Evaluation of cytogenetic effects of isoproturon on the root meristem cells of Allium sativum L. Biomed. Environ. Sci., 14: 214-219.
- Chopra, A.K., C. Pathak and G. Prasad, 2009. Scenario of heavy metal contamination in agricultural soil and its management. J. Appl. Nat. Sci., 1: 99-108.
- Liu, D., I. Kottke and D. Adam, 2007. Localization of cadmium in the root cells of Allium cepa by energy dispersive x-ray analysis. Biol. Plant., 51: 363-366.
- Liu, D., P. Xue, Q. Meng, J. Zou, J. Gu and W. Jiang, 2009. Pb/Cu effects on the organization of microtubule cytoskeleton in interphase and mitotic cells of Allium sativum L. Plant Cell Rep., 28: 695-702.
- Opdenakker, K., T. Remans, E. Keunen, J. Vangronsveld and A. Cuypers, 2012. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in mapkinase transcript levels. Environ. Exp. Bot., 83: 53-61.
- Fiskesjo, G., 1985. The Allium test as a standard in environmental monitoring. Hereditas, 102: 99-112.
- Abedin, M.J. and A.A. Meharg, 2002. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil, 243: 57-66.
- Zou, J., J. Yue, W. Jiang and D. Liu, 2012. Effects of cadmium stress on root tip cells and some physiological indexes in Allium cepa var. agrogarum L. Acta Biol. Cracoviensia Bot., 54: 129-141.
How to Cite this paper?
APA-7 Style
Chatterjee,
S., Chatterjee,
S. (2021). Effect of Arsenic on Growth and Cell Division in Root Tip Cells of (Allium sativum L.). Asian Journal of Emerging Research, 3(2), 109-113. https://doi.org/10.3923/ajerpk.2021.109.113
ACS Style
Chatterjee,
S.; Chatterjee,
S. Effect of Arsenic on Growth and Cell Division in Root Tip Cells of (Allium sativum L.). Asian J. Emerg. Res 2021, 3, 109-113. https://doi.org/10.3923/ajerpk.2021.109.113
AMA Style
Chatterjee
S, Chatterjee
S. Effect of Arsenic on Growth and Cell Division in Root Tip Cells of (Allium sativum L.). Asian Journal of Emerging Research. 2021; 3(2): 109-113. https://doi.org/10.3923/ajerpk.2021.109.113
Chicago/Turabian Style
Chatterjee, Sabyasachi, and Soumik Chatterjee.
2021. "Effect of Arsenic on Growth and Cell Division in Root Tip Cells of (Allium sativum L.)" Asian Journal of Emerging Research 3, no. 2: 109-113. https://doi.org/10.3923/ajerpk.2021.109.113

This work is licensed under a Creative Commons Attribution 4.0 International License.



