Health Risk Assessment of the Urban Stretch, Lower Niger Rivers with References to Aquatic Biota and Anthropoids
| Received 25 Feb, 2021 |
Accepted 27 Apr, 2021 |
Published 15 Jun, 2021 |
Background and Objective: The lower Niger River at Swale does not serve as a source of water to human-only but also to anthropoids. However, the river is drained by many hazardous pollutants due to anthropogenic activities. Therefore, the occurrence of priority hazardous Polycyclic Aromatic Hydrocarbons (PAHs) in water, suspended particulates matter, and bottom sediments of the urban stretch of the rivers at the Swale market was investigated. Materials and Methods: Thirty-six samples were collected monthly, comprises of surface water, suspended particles and bottom sediments were collected monthly. Water samples was extracted using Liquid-liquid extraction method, using a mixture of dichloromethane and n-hexane, while soxhlet extraction was used for the extraction of suspended particles and sediment samples. The samples were analyzed by means of gas chromatography/mass spectrometry. One-way analysis of variance was used to compare the significance of the differences of total PAHs between different matrixes and seasonal variation. Results: The mean Concentration of Total PAHs and seasonal distribution in the surface water suspended particles and bottom sediments of the lower Niger Delta Rivers, Nigeria at Swale varies significantly between the seasons and the matrixes (p < 0.05, F = 7.30). Higher concentrations were observed in the wet season than the dry season in all the matrixes, with the bottom sediments having the highest concentrations (7270±4.10 µg/gdw), followed by suspended particles (1320±7.80 µg/gdw), while the surface water had the least (357.90±2.20 µg/L). Conclusion: The high concentrations recorded in these matrixes is that the aquatic organisms can bioaccumulates along the food chain. The pollution load of PAHs in this lower Niger river at the Swale market must therefore be reduced to improve the health of ecosystems, thereby guaranteeing safe human consumption of fish and other aquatic organisms.
INTRODUCTION
According to the most recent surveys on national water quality from the United State Environmental Protection Agency, nearly half of our rivers, streams, and more than one-third of our lakes are polluted and unfit for swimming, fishing, and drinking1 A wide range of wastewater is dumped largely untreated into the environment, polluting the rivers, lakes, estuarine, and marine ecosystem. These include Polychlorinated biphenyls (PCBs), Polycyclic Aromatic Hydrocarbons (PAHs), Dichlorodiphenyltrichloroethane (DDT), heterocyclic compounds (such as pyridines or quinolines), pharmaceutical substances, radionuclides, and heavy metals. Others such as residues of pesticides, herbicides, and germicides have been implicated in aquatic pollution2. Among these hazardous compounds, PAHs represent a unique class because they are among the most recalcitrant3. These substances are known to exhibit toxic, mutagenic, and carcinogenic effects on the biotic components of the environment including man4.
Consequently, PAHs have been labelled as ‘priority pollutants to be monitored in industrial effluents, natural waters, soil, sediment, and food5.
These hydrocarbons are derived from the incomplete combustion of organic matters including coal, oil, gas, diesel, tar, wood, garbage, or other organic substances such as tobacco and charbroiled meat are a class of toxic environmental pollutant that has accumulated in the environment due to a variety of anthropogenic activities6. They can also be introduced into the environment through volcanoes, forest fires, automobile exhaust, domestic wood burning, thermal decomposition, cigarette smoking, and other combustibles and subsequently found throughout the environment including air, soil, and water7.
Numerous PAH compounds are found in our environment; however, analysis of PAH is limited to a few compounds, generally, the 16 priority compounds listed by US EPA as potentially toxic8. Although a lot of research on PAHs have been published in developed countries as a result of the negative effects, they have on the biota especially human health, little information is available in developing countries such as Nigeria9,10,11.
The lower Niger rivers at Swale is drained by municipal and industrial solid and liquid wastes, abattoirs and automobile exhaust especially from the dredging companies which must have contributed to the sordid state of the rivers. Therefore, the objective of this investigation is to assess the quality of the rivers with references to PAHs in the water, suspended particles and the bottom sediment. This becomes inevitable because besides serving downstream inhabitants for different purposes such as vegetable farming and domestic usage, the water from the rivers are used in the abattoirs for washing meats, fishes and cleaning of the abattoirs.
MATERIALS AND METHODS
Study Area: The study area lies between latitudes 40 53’42.9576 and longitude 60 16’39.7164”. The lower Niger River at Swale is at the end of the major market in Bayelsa state Nigeria. Inevitably is at the receiving ends of all the pollutants that emanate from the market and besides, it also drains by municipal and industrial solid and liquid wastes, abattoirs and automobile exhausts.Chemicals and Reagents: All the reagents used for these analyses are of analytical grade and are products of Eagle Scientific Limited, England, BDH Limited, England, Surechem Products Ltd, England, Riedel-De Haen, Germany, Sigma-Aldrich, Germany, Kermel, China, and Burgoyne Burbridges, India.
Samples Collection and Extraction: Samples were collected monthly from November 2018 to October 2019 and were taken from point of drains to the rivers at Swale market and were visited monthly. PAHs in water were extracted using Liquid-liquid extraction as described by Nieuwoudt et al.12, Okedeyi et al.13. An equal volume of water was saturated with 75 g NaCl and transferred to a separating funnel and extracted thrice using a mixture of dichloromethane and n-hexane. The mixture was vigorously shaking and the extract was dehydrated using anhydrous sodium sulfate, and concentrated to 2.0 mL using a rotary evaporator at 40°C, and reconstituted using 1 mL ofn-hexaneand analyzed using gas chromatography/mass spectrometry (GC-MS).
Soxhlet extraction was used for the extraction of suspended particles and sediment samples14. Ten grams of powdered samples were extracted in a Soxhlet apparatus for 15 hours with 50 ml of dichloromethane: methanol (v \ v 3:1) mixture. The extract was then reduced to 20 mℓ in a water bath at 45°C and transferred to a 100 mℓ separating funnel and 20 mℓ distilled water added. The mixture was washed with three 10 mℓ portions of 0.1 M Na2CO3 and reduced by evaporation and re-dissolved in 1 mℓ n-hexane. The extract was finally reduced to 1 mL under a gentle stream of nitrogen gas. All sample extracts were kept in amber glass vials at −4°C until analysis. The extraction was done in triplicate.
Sample Analysis: The extract (1 μL) was subjected to qualitative and quantitative analysis on GC/MS Agilent 7890/5975C. The following analytical conditions were used: capillary column – HP- 5MS (30 m, 0.32mm 25 microns), rate of the carrier gas – 1,2 cm3/min, input method – PTV, injector temperature – 300°C, temperature program – initial temperature70°C, 1 min, heating rate – 10°C*min1, first isotherm – 190°C for 1 min, heating rate – 4°C*min1, second isotherm – 280°C for 20 min, ionization – E +70 eV, – data collection method – SIM, interface temperature – 285°C, temperature of source – 230°C, the temperature of Quadrupole – 150°C. The injector was held at 200°C and the FID was maintained at 300°C.
An external standard calibration comprising 18 PAH standards was used to determine the identity and quantity of each component peak in the sample chromatogram.
Human Health Risk Assessment: The residual level of PAHs in water,
Risk Assessment of PAHs in Swale Rivers: The risks to the user of the investigated Rivers was determined by the estimation of:
- The toxic equivalent quotient (TEQ)
- Average daily dosage by dermal contact (ADD dermal) in mg/kg/day
- Hazard quotient (HQ)
- Incremental lifetime cancer risk (ILCR) and
- Risk index (RI) in the water
Toxic equivalent quotient (TEQ): The toxic equivalent quotient (TEQ) is the carcinogenic potential of the high molecular weight PAHs and is calculated by multiplying their toxic equivalent factor (TEF) with the mean concentration of each PAH in the sediment15,16,17 as shown in equation (i). The PAHs is calculated to benzo[a]pyrene, which is the most carcinogenic PAH.
Where Ci = concentration of individual PAHs,
TEFi =toxic equivalent factor relative to benzo(a)pyrene
Average Daily Dosage by Dermal Contact (ADD dermally)
To obtain the average dosage by dermal contact, the following relationship was employed
| ADDderm = | C*SA*Kp*ET*ED*CF BW * AT |
…………………………………………………equation (ii) |
Where C = the pollutant’s concentration in the water sample (mg/L)
SA = the exposed skin area (18,000 cm2).
Kp (cm/h) = the dermal permeability coefficient for PAHs congener (possibly cancerous), (BaA: 8.10E−01; Chry: 8.10E−01; BbF:1.20E+00; BaP: 1.20E+00; DiahA: 2.70E+00; InPy: 1.90E+00; Nap: 6.90E−02; Phe: 2.70E−01; Flt: 3.60E−01;)
ET = the exposure time of bathing (0.58 h/day)
ED = stands for the exposure duration (30 years)
CF = stands for unit conversion factor (L/1000 cm)
BW = the average body weight (adults: 7kg; children: 15kg)
AT = the average time. i.e ED *365 days (10,950 days)
Hazard Quotient and Hazard Index: The hazard quotient (HQ) is the ratio of the ADD to the reference dose (RfD) for each contaminant18 while hazard index (HI) was calculated as the sum of the HQs for all the congeners in each sample as shown19.
Where RfD = Dermal reference dose for each PAHs congener and was only available for 6 of the 16 priority PAHs
The probability of a human developing cancer over a lifetime as a result of exposure to a suspected carcinogen is termed ILCR. Therefore, ILCR and RI were calculated for the carcinogenic PAHs using Equations (iv) and (v) in agreement with US EPA guidelines20
Where
CSF = cancer slope factor for individual congener and CSF for BaP is 7.3 mg/kg/day14. The slope factors for other PAHs were thereafter estimated from that of BaP by multiplying the value with the respective toxic equivalent factor (TEF) for each of the congeners18.
Statistical Analysis: One-way analysis of variance and Tukey's HSD multiple comparison test were used to compare the significance of the differences of total PAHs between different matrixes (water, suspended particulates matter, and bottom sediments), and seasonal variation using SPSS version 15 for Windows.
RESULTS
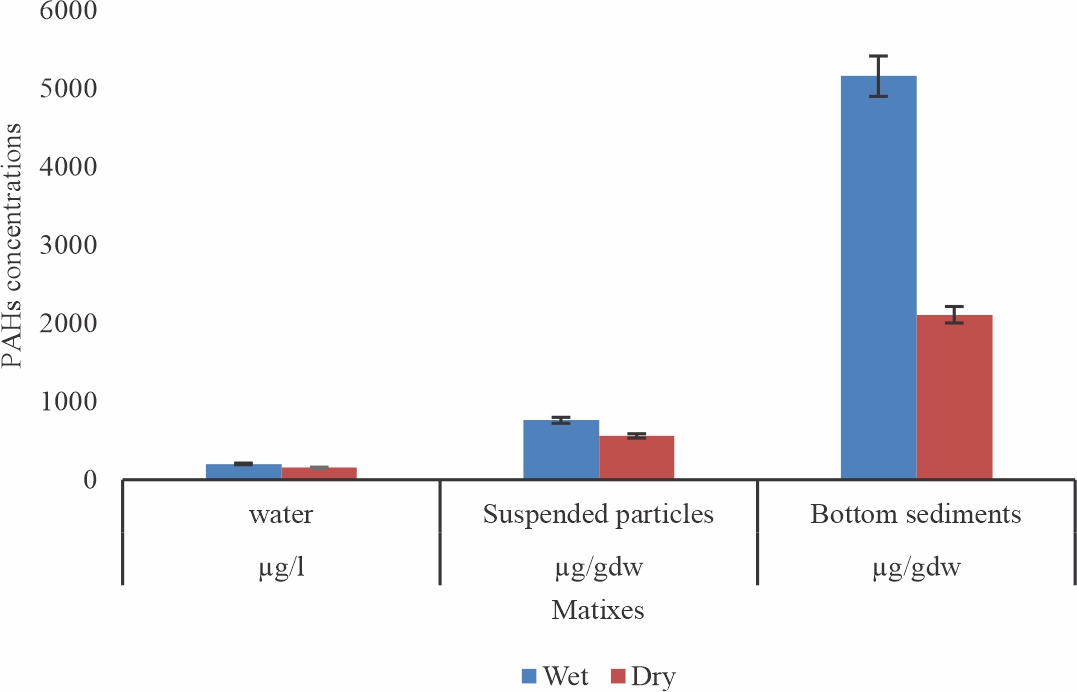
Distribution of PAHs in Matrixes: The mean Concentration of Total PAHs and seasonal distribution in the surface water suspended particles and bottom sediments of the lower Niger Delta Rivers, Nigeria at Swale is shown in Table 1, with further illustration in Fig. 1. The concentrations of the PAHs vary significantly between the seasons and the matrixes (p<0.05, F = 7.30).
|
|||||
| Matrixes | Water( μg/l) | Suspended particles (μ/gdw) | Bottom sediments (μ/gdw) | ||
| WET SEASON | 2 03.70 ±3. 20a | 760.00 ± 5.40 b | 5160.00 ± 7.10 c | ||
| DRY SEASON | 154.20 ±1 .70 b | 560.00 ± 4.10 c | 2110.00 ± 5.90 d | ||
| TOTAL PAHs | 357.90 ± 2.20 k | 1320.00 ± 7.80 n | 7270.00 ± 4.10 m | ||
Mean with a different superscript in the row and the column are significantly different (p<0.05) |
|||||
Though there are numerous PAH congeners in the environment, in this study, only thirteen were determined in the course of this investigation (Table 2 & 3).
Bottom sediments had the highest concentrations (7270±4.10 μg/gdw), followed by suspended particles (1320± 7.80 μg/gdw), while the surface water had the least (357.90±2.20 μg/L).
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
Seasonally, higher concentrations were observed in the wet season in all the matrixes; In the water column: 2 03.70±3. 20 and 154.20±1.70 (μg/l) for wet and dry season respectively; suspended particles had 760±5.40 and 560±4.10 (μ/gdw) and the values for bottom sediments were 5160±7.10 and 2110±5.90 (μ/gdw) respectively (Table 1).
Dibenzothiophene was the least recorded individual PAHs in all the matrixes (6.70±1 .80 μg/l) and was observed in the water (Table 3), while fluoranthene was the highest PAHs congener observed (265.20 ± 2.10 μ/gdw), and was recorded in the bottom sediments (Table 2).
|
|||||
| Matrixes | Water (μg/l) | Suspended particles (μg/gdw) | Bottom Sediments (μg/gdw) | ||
| Naphthalene | 43.20± 1.30 | 56.50 ± 2.20 | 78.10 ± 3.50 | ||
| Acenaphthylene | 21.60 ±0.10 | 33.50 ± 2.30 | 46.40± 0.30 | ||
| Acenaphthene | 14.40 ± 0.50 | 19.20 ± 0.10 | 31.40 ± 0.10 | ||
| Azulene | 66.10 ± 2.10 | 72.80± 1.30 | 165.10± 3.20 | ||
| Fluorene | 17.10 ± 0.30 | 21.20 ± 0.30 | 37.60 ± 0.30 | ||
| Phenanthrene | 101.40 ± 5.20 | 136.70 ± 8.20 | 152.70 ± 7.50 | ||
| Anthracene | 19.20 ± 2.10 | 23.50 ± 1.80 | 32.80 ± 2.10 | ||
| Pyrene | 201.10 ± 2.90 | 268.90 ± 2.10 | 291.70 ± 4.30 | ||
| Fluoranthene | 216.60 ± 1.10 | 230.40 ± 1.30 | 265.20 ± 2.10 | ||
| Benzo[b]fluoranthene | 80.00± 2.10 | 101.10± 1.50 | 160.20 ± 3.10 | ||
| Indene | 68.00 ± 1.10 | 103.40 ± 2.20 | 142.20 ± 7.20 | ||
| Dibenzothiophene | 7.10 ± 2.50 | 11.20 ± 0.40 | 16.00± 1.10 | ||
| Benzo[a]pyrene | 101.20± 1.20 | 117.60± 2.10 | 130.20± 3.20 | ||
|
|||||
| Matrixes | Water (μg/l) | Suspended particles (μg/gdw) | Bottom Sediments (μg/gdw) | ||
| Naphthalene | 35.20 ± 0.50 | 48.50 ± 1.40 | 62.10 ± 2.10 | ||
| Acenaphthylene | 15.60 ± 0.80 | 24.50 ± 0.20 | 32.40 ± 0.50 | ||
| Acenaphthene | 09.20 ± 0.10 | 11.10 ± 0.40 | 27.20 ± 0.30 | ||
| Azulene | 44.10 ± 1.30 | 52.30± 1.10 | 85.30± 2.20 | ||
| Fluorene | 12.60 ± 0.70 | 19.10 ± 0.20 | 30.40 ± 0.10 | ||
| Phenanthrene | 81.20 ± 1.60 | 116.10 ± 5.10 | 122.20 ± 3.40 | ||
| Anthracene | 16.10 ± 1.40 | 19.20 ± 1.20 | 32.80 ± 1.90 | ||
| Pyrene | 107.10 ± 1.10 | 119.20 ± 1.40 | 291.70 ± 2.30 | ||
| Fluoranthene | 176.70 ± 4.10 | 201.20± 2.20 | 233.70 ± 1.40 | ||
| Benzo[b]fluoranthene | 60.70± 2.20 | 77.10 ± 1.30 | 102.60 ± 3.10 | ||
| Indene | 63.10± 4.0 | 80.70 ± 2.10 | 103.50± 1.90 | ||
| Dibenzothiophene | 6.70±1 .80 | 8.20 ± 2.20 | 12.50± 2.50 | ||
| Benzo[a]pyrene | 69.80± 0.50 | 107.00± 3.20 | 118.50± 2.10 | ||
Human Health Risk Assessment: The hazard to the user of the Swale rivers as a result of exposure to PAHs found in the water by dermal contact was assessed. The toxic equivalent quotient (TEQ) of PAHs of the sediments of the investigated Rivers revealed that the wet season had higher TEQ (266.399 μg/gdw), than the dry season (234.691 μg/gdw) and the annual PAHs reported in the investigation year was 250.545±5.40 μg/gdw (Table 4). The TEQ was calculated for the 13 PAHs showed Benzo[a]pyrene was the most toxic (130.200), PAHs, followed by Benzo[b]fluoranthene (82.080), and Fluoranthene (21.216). The trio contributed roughly 98% of the total TEQ in the samples analyzed. The other PAH congeners were very negligible.
The hazard quotients (HQs), and the probability of a human developing cancer over a lifetime as a result of exposure to PAHs (RI) in Swale River, lower Niger Delta Nigeria is shown in Table 5. The HQs for the congeners that are probable carcinogenic are less than 1 (Table 5).
|
|||||
| PAHs | TEF | Seasons | |||
| Wet | Dry | ||||
| Naphthalene | 0.001 | 0.078 | 0.062 | ||
| Acenaphthylene | 0.001 | 0.046 | 0.032 | ||
| Acenaphthene | 0.001 | 0.031 | 0.027 | ||
| Azulene | 0.0005 | 0.082 | 0.042 | ||
| Fluorene | 0.001 | 0.037 | 0.030 | ||
| Phenanthrene | 0.001 | 0.152 | 0.122 | ||
| Anthracene | 0.01 | 0.328 | 0.328 | ||
| Pyrene | 0.001 | 0.291 | 0.291 | ||
| Fluoranthene | 0.08 | 21.216 | 18.696 | ||
| Benzo[b]fluoranthene | 0.80 | 82.08 | 128.16 | ||
| Indene | 0.001 | 0.142 | 0.103 | ||
| Dibenzothiophene | 0.0005 | 0.008 | 0.006 | ||
| Benzo[a]pyrene | 1.0 | 130.20 | 118.5 | ||
| ∑TEQ (μg/gdw) | 266.399 | 234.691 | |||
|
|||||||
| PAHs | RFD | ADD | HQ | CSF | ILCR | ||
| Anthracene | 0.02 | - | 0.02 | 0.073 | - | ||
| Benzo(g,h,i)perylene | 0.04 | - | 0.04 | 5.840 | - | ||
| Fluoranthene | 0.04 | 0.009 | 0.22 | 0.584 | 0.005 | ||
| Fluorene | 0.04 | - | 0.04 | 0.0073 | - | ||
| Naphthalene | 0.04 | 0.004 | 0.10 | 0.0073 | 0.00003 | ||
| Phenanthrene | 0.04 | 0.007 | 0.18 | 0.0073 | 0.00005 | ||
| HI | 0.60 | ||||||
| RI | 0.00508 | ||||||
RfD = Dermal reference dose; ADD= Average Daily Dosage by Dermal Contact; CSF = cancer slope factor; ILCR = the incremental probability of a person to develop cancer over a lifetime as a result of exposure to a possible carcinogen |
|||||||
DISCUSSION
The distribution of PAHs in water, suspended particles and bottom sediment of the lower Niger rivers at Swale market were above the WHO maximum permissible limit of 10 μg/l in water and 0.01 μg/gdw in suspended particles and sediments19. The concentrations observed in these matrixes were comparable to the reported values in the surface waters, sediment, and biota of an oil-polluted mangrove wetland in the Niger Delta Nigeria20. Similar observations were reported by Li and Zhang21, Clinton et al.22 in Tema Habour and the coastal belt of Ghana respectively and by Essumang23 in the estuarine in Malaysia. Contrarily, the concentrations of PAHs examined in water and sediment from Badagry Creek and Ologe Lagoon, Lagos Nigeria was far below our reported values24. The high total PAH’s concentrations observed in this investigation could be attributed to input from domestic wastes, discharge of sewage, oil leakage, dredging activities, asphalt road surface erosion, motor vehicle emissions, oil leakage, drifts from oil-polluted areas, and other activities. The concentrations of PAHs reported in this investigation were within the range previously reported elsewhere in Nigeria, Niger Delta creeks10.
The sediment loads of PAHs for both seasons consistently higher than that of suspended particles and the surface water, apart from being a better indicator of pollution, it also serves as a potential25 reservoir for organic compounds that are resistant to biodegradation in the aquatic environment. Similarly, the hydrophobic nature of PAH’s as they tend to adsorb on the surface sediments because they are insoluble in the water column 26.
Seasonal variation revealed that the concentrations of the PAHs were higher in the dry season than in the wet season. This can be attributed to the fact that variation in concentrations of organic pollutants is influenced by seasonal changes27. Strong winter winds and wildfires, and the number of vehicles on the roads are other factors affecting PAH levels in surface water and sediments28. Similarly, unique air circulation and fluctuation of tides and waves also influence the deposition of PAHs in water and sediment as observed in this investigated region27.
When the sediments concentrations of PAH congeners were compared with US National Oceanic sediment guidelines, it revealed that they were slightly above the effect range medium. (ERM), an indication of the likelihood of high negative effect on biota and rivers dependents in the lower Niger rivers at Swale market28.
The HQs of the lower Niger Delta rivers at Swale market to exposure of the users to the contaminants in the surface and bottom water through skin absorption were all lesser than 1 as shown in Table 5, an indication that there would be no possibility of any non-carcinogenic threat as due to dermal contact. It is important to note that when HQ or HI is less than 1, it indicates no significant non-carcinogenic risk, while the effect will be considered significant when the value exceeds 129. Similarly, HI, which is the sum of HQs of the congeners was less than 1 and was far below the United States Environmental Pollution Agency’s recommended limit1,30.
The probability of a person developing cancer over a lifetime as a result of exposure to a possible carcinogen is termed ILCR. According to US EPA, the acceptable ILCR of one in a million populations (1 × 10−6) is considered acceptable, while 1 × 10−5 but lesser than 1 × 10−4 may although be tolerable but not recommendable, and values up to 1 × 10−4 or higher portend serious cancer risk in humans34. The ILCR of Fluoranthene and Phenanthrene of lower Niger rivers was 5 × 10−5, which is tolerable, while Naphthalene (3 × 10−5) poses a cancer risk to the user of the water. The RI (sum of ILCRs) by dermal absorption is 5.08× 10−3, indicating a very serious cancer risk. The carcinogenic effects of PAHs have been observed in certain parts of the body especially the skin, gastro-intestine, liver, lung, and bladder7.
The TEQs observed for Benzo[a]pyrene, Fluoranthene and Benzo[b]fluoranthene in this investigation signified a possible cancer risk to those who may be exposed to the bottom sediment31,32, meanwhile, other congeners did not record any significant contribution, hence the potential risk due to cPAHs in lower Niger rivers sediment at Swale market may not pose a serious risk to the people.
CONCLUSION
This finding had shown that the lower Niger rivers at the Swale market are contaminated which could be attributed to both anthropogenic and natural factors. The health risk assessment showed that there was no possibility of non-carcinogenic risk in the matrixes. However, the TEQs for some PAHs was very high, an indication of possible cancer risk. Nonetheless, our rivers need to be kept clean, all activities that likely increase pollution in this major river in the Niger Delta ecosystem should be avoided for the safety of aquatic biota and human beings.
SIGNIFICANCE STATEMENT
The lower Niger River serves as a source of water to aquatic organisms and the terrestrial alike, hence investigating the health status becomes inevitable. The presence of PAHs in the surface water, suspended particles and bottom sediments can leads to the bottom feeders accumulating PAHs in their muscles, and subsequently, bioaccumulates along the food chain, which may have negative impact on the ecosystem, especially human, and being at the top of the trophic level. PAHs persist, toxic, carcinogenic, and mutagenic, hence effort should be made to curtail its input into aquatic habitat. This study has also revealed that the level of inputs of pollutants into aquatic habitat is seasonal dependent with wet season having high affinity cause by anthropogenic activities. The concentrations of PAHs in the matrixes were higher in the wet season than the dry season which may be because of high drains of the rivers as a result of runoff from the market’s wastes and municipal sewage wastes.
REFERENCES
- Bawuro, A.A., R.B. Voegborlo and A.A. Adimado, 2018. Bioaccumulation of heavy metals in some tissues of fish in lake Geriyo, Adamawa state, Nigeria. J. Environ. Public Health.
- Ikpesu, , T.O. and A.B. Ariyo, 2017. Evaluation of hydrochemical and microbiological contaminants isolated from brass river in Niger-Delta ecological zone. J. Sci. Technol., 9: 20-30.
- Arulazhagan, P., N. Vasudevan and I.T. Yeom, 2010. Biodegradation of polycyclic aromatic hydrocarbon by a halotolerant bacterial consortium isolated from marine environment. Int. J. Environ. Sci. Technol., 7: 639-652.
- Abubakar, A., A. Uzairu, P.A. Ekwumemgbo and O.J. Okunola, 2015. Risk assessment of heavy metals in imported frozen fish Scomber scombrus Species sold in Nigeria: A case study in Zaria metropolis. Adv. Toxicol.
- Kanaly, R.A and S. Harayama, 2000. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol., 182: 2059-2067.
- Rey-Salgueiro, L., M.S. Garcia-Falcon, E. Martinez-Carballo and J. Simal-Gandara, 2008. Effects of toasting procedures on the levels of polycyclic aromatic hydrocarbons in toasted bread. Food Chem., 108: 607-615.
- Aljerf, L., 2018. A gateway to metal resistance: bacterial response to heavy metal toxicity in the biological environment. Ann. Adv. Chem., 2: 32-44.
- Juhasz, A.L. and R. Naidu, 2000. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo[a]pyrene. Int. Biodeterior. Biodegrad., 45: 57-88.
- Akwetey, W. D. Eremong and A. Donkoh, 2013. Chemical and nutrient composition of cattle hide ("Welle") using different processing methods. J. Anim. Sci. Adv., 3: 176-180.
- Perschbacher, P.W., 2005. Temperature effects on acute copper toxicity to juvenile channel catfish Ictalurus punctatus. Aquaculture, 243: 225-228.
- Ogunfowokan, A.O., O.I. Asubiojo and O.S. Fatoki, 2003. Isolation and determination of polycyclic aromatic hydrocarbons in surface runoff and sediments. Water, Air, Soil Pollut., 147: 245-261.
- Nieuwoudt, C., R. Pieters, L.P. Quinn, H. Kylin, A.R. Borgen and H. Bouwman, 2011. Polycyclic aromatic hydrocarbons (PAHs) in soil and sediment from industrial, residential, and agricultural areas in central south Africa: an initial assessment. Soil Sediment Contam.: An Int. J., 20: 188-204.
- Okedeyi, O.O., M.M. Nindi, S. Dube and O.R. Awofolu, 2012. Distribution and potential sources of polycyclic aromatic hydrocarbons in soils around coal-fired power plants in south Africa. Environ. Monit. Assess., 185: 2073-2082.
- Rezaee, M., Y. Assadi, M.R.M. Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, 2006. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A, 1116: 1-9.
- El-Beqqali, A., A. Kussak and M. Abdel-Rehim, 2006. Fast and sensitive environmental analysis utilizing microextraction in packed syringe online with gas chromatography–mass spectrometry. J. Chromatogr. A, 1114: 234-238.
- Lerda, D., 2011. Polycyclic aromatic hydrocarbons (PAHs) Factsheet, 4th Edn. JRC Technical Notes, Italy.
- Nekhavhambe, T., T.V. Ree and O. Fatoki, 2014. Determination and distribution of polycyclic aromatic hydrocarbons in rivers, surface runoff, and sediments in and around Thohoyandou, Limpopo province, South Africa. Water SA.
- Wei, H., Z. Le, L. Shuxian, W. Dan, L.Xiaojun, J. Lan and M. Xiping, 2015. Health risk assessment of heavy metals and polycyclic aromatic hydrocarbons in soil at coke oven gas plants. Environ. Eng. Manag. J., 14: 487-496.
- Jamhari, A.A., M. Sahani, M.T. Latif, K.M. Chan, H.S. Tan, M.F. Khan and N.M. Tahir, 2014. Concentration and source identification of polycyclic aromatic hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmosp. Environ., 86: 16-27.
- Kumar, A., B.S. Bisht, V.D. Joshi and T. Dhewa, 2011. Review on bioremediation of polluted environment: A management tool. Int. J. Environ. Sci., 1: 1079-1093.
- Li, S. and Q. Zhang, 2010. Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the upper Han river, China. J. Hazard. Mater., 181: 1051-1058.
- Clinton, H.I., G.U. Ujagwung and M. Horsfall, 2009. Evaluation of total hydrocarbon levels in some aquatic media in an oil polluted mangrove wetland in the Niger delta. Applied Ecol. Environ. Res., 7: 111-120.
- Essumang, D.K., 2010. Distribution, levels and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in some water bodies along the coastal belt of Ghana. Scient. World J., 10: 972-985.
- Zakaria, M.P., H. Takada, S. Tsutsumi, K. Ohno, J. Yamada, E. Kouno and H. Kumata, 2002. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs. Environ. Sci. Technol., 36: 1907-1918.
- Hezhong, Y., Z. Enlou, L. Qi, W. Rong and L. Enfeng, 2015. Sources appointment and ecological risk assessment of polycyclic aromatic hydrocarbons (pahs) in sediments of Erhai lake, a low-latitude and high-altitude lake in southwest China. Environ. Sci. Pollut. Res., 23: 4430-4441.
- Kucuksezgin, F., A. Kontas, O. Altay, E. Uluturhan and E. Dar?lmaz, 2006. Assessment of marine pollution in Izmir bay: nutrient, heavy metal and total hydrocarbon concentrations. Environ. Int., 32: 41-51.
- Kannan, K., B. Johnson-Restrepo, S.S. Yohn, J.P. Giesy and D.T. Long, 2005. Spatial and temporal distribution of polycyclic aromatic hydrocarbons in sediments from inland lakes in Michigan. Environ. Sci. Technol., 39: 4700-4706
- Long, E.R. and D.D. Macdonald, 1998. Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Hum. Ecol. Risk Assess. Int. J., 4: 1019-1026.
- Titilawo, Y., A. Adeniji, M. Adeniyi and A. Okoh, 2018. Determination of levels of some metal contaminants in the freshwater environments of Osun state, southwest Nigeria: a risk assessment approach to predict health threat. Chemosphere, 211: 834-843.
- Hussein, R., K. Al-Ghanim, M. Abd-El-Atty and L. Mohamed, 2016. Contamination of red sea shrimp (Palaemon serratus) with polycyclic aromatic hydrocarbons: a health risk assessment study. Pol. J. Environ. Stud., 25: 615-620.
- Salem, D.M.S.A., F.A.E.M. Morsy, A.E. Nemr, A. El-Sikaily and A. Khaled, 2014. The monitoring and risk assessment of aliphatic and aromatic hydrocarbons in sediments of the Red Sea, Egypt. Egypt. J. Aquat. Res., 40: 333-348.
- Rábago-Castro, J.L., J.G. Sanchez, R. Pérez-Castañeda and A. González-González, 2006. Effects of the prophylactic use of romet®-30 and copper sulfate on growth, condition and feeding indices in channel catfish (Ictalurus punctatus). Aquaculture, 253: 343-349.
How to Cite this paper?
APA-7 Style
Chinonye,
E.O., Ohwofasa,
I.T. (2021). Health Risk Assessment of the Urban Stretch, Lower Niger Rivers with References to Aquatic Biota and Anthropoids. Asian Journal of Emerging Research, 3(2), 104-108. https://doi.org/10.3923/ajerpk.2021.104.108
ACS Style
Chinonye,
E.O.; Ohwofasa,
I.T. Health Risk Assessment of the Urban Stretch, Lower Niger Rivers with References to Aquatic Biota and Anthropoids. Asian J. Emerg. Res 2021, 3, 104-108. https://doi.org/10.3923/ajerpk.2021.104.108
AMA Style
Chinonye
EO, Ohwofasa
IT. Health Risk Assessment of the Urban Stretch, Lower Niger Rivers with References to Aquatic Biota and Anthropoids. Asian Journal of Emerging Research. 2021; 3(2): 104-108. https://doi.org/10.3923/ajerpk.2021.104.108
Chicago/Turabian Style
Chinonye, Ezenwaka, Oluchi, and Ikpesu Thomas Ohwofasa.
2021. "Health Risk Assessment of the Urban Stretch, Lower Niger Rivers with References to Aquatic Biota and Anthropoids" Asian Journal of Emerging Research 3, no. 2: 104-108. https://doi.org/10.3923/ajerpk.2021.104.108

This work is licensed under a Creative Commons Attribution 4.0 International License.




