Biochemical and Toxicological Evaluation on Tilapia guineensis Exposed to Produced Formation Water
| Received 26 Dec, 2020 |
Accepted 02 Mar, 2021 |
Published 09 Jun, 2021 |
Background and Objectives: To evaluate the acute effects of Produced Formation Water on the oxidative stress and biochemical parameters of Tilapia guineensis under semi-static conditions at a concentration of 100 to 700ml for 96 hours. Materials and Methods: The fishes were exposed to different concentration of Produced Water, and their LC50 value of 223.10 ml was calculated after 96 hour exposure period. The antioxidant enzyme profile investigated include: glutathione reductase, superoxide dismutase, glutathione peroxidase and catalase. Stress biomarkers such as protein, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) were also determined at 24 hour, 48 hour, 72 hour and 96 hour exposure periods. Results: Lipid peroxidation (MDA) showed significant (p<0.05) decreases in higher concentration and exposure period of 72 hour and 96 hour. Significant concentration-dependent increases (p<0.05) were observed in the liver function enzymes, Superoxide dismutase, Catalase and glutathione reductase when compared to the control. Glutathione peroxidase when compared to the control decreased significantly (p<0.05) in higher concentrations and 48-96 hour. Conclusion: The changes in the hepatic antioxidant enzyme activities and serum metabolites were evident of oxidative damage induced by Produced Formation Water. This showed that Produced water is toxic to the fish and regulatory agency in oil and gas industry should improve their effort to ensure they are properly treated before discharged into the aquatic ecosystems to prevent potential toxicity associated with it.
INTRODUCTION
Fish are valuable in monitoring aquatic toxicity of a particular toxicants as they possess the same biochemical pathways as mammalian species does, to deal with the toxic effects of endogenous and exogenous agents1. These defenses include low molecular weight free radical scavengers such as reduced glutathione (GSH), and enzymatic defense such as superoxide dismutase (SOD), Catalase and glutathione-s-transferases (GST)2. Subsequently, there is evidence that fish have the potential to serve as suitable model organisms for studying the mechanistic aspects of oxidative toxicity such as chemical carcinogenesis and damage repair that are also relevant to biomedical toxicology3. Several fish studies have demonstrated the importance of antioxidant defenses in protecting cells and organisms from oxidative damage and toxicity4. In addition to protecting internal systems, some of the low molecular weight scavengers and enzymatic defenses are responsive to challenge with compounds that cause oxidative damage5, representing an adaptive consequence of exposure6. Due to the potential for reactive oxygen species (ROS) to damage tissues and cellular components such as proteins and membranes7, oxidative stress has become significant in both mammalian and aquatic toxicology8.
The hazardous compounds they accumulate in their tissues are directly or indirectly consumed by humans, and are capable of transforming xenobiotic compounds into carcinogenic and mutagenic metabolites9.
Exposure of fish to contaminants often results in changes in environmental impacts and harmful effects to organisms, when aquatic ecosystems are impacted10. The mechanisms at which fish evolved to cope with these changes could be physiological, behavioural, and biochemical responses which they use to maintain their normal, healthy homeostasis despite the adverse environmental condition11. These are usually termed “Stress responses’’ to environmental changes. When there is an overstimulation of these stress responses, mainly of anthropogenic origin, over the capacity of the animal to resume normal life activities, it may lead to severe ecological disturbances12. The activation of these stress responses can then be used as biomarkers at the organismal level to study the possible effects of physiological constraints or changes of habitat quality on the individual organism13. Free radicals may overwhelm the systems of exposed fishes resulting in oxidative stress, which is an important source of gene mutation and cellular injury14. The majority of these radicals involves oxygen and is referred to as reactive oxygen species (ROS)15. Antioxidant responses can be an important part of biomontoring studies, as many xenobiotics are pro-oxidants that exert their effects through induction of oxidative stress. Antioxidant enzymes are however often less responsive than other biomarkers to exposure and may greatly differ in expression levels in different species16. Therefore, when investigating the effects of xenobiotics such as produced water effluents on biota, antioxidant biomarkers can be very useful as part of a much larger range of biomarkers being used. Theses may include oxidative DNA damage, oxidative damage products like protein carbonyl, or lipid peroxidation products such as MDA and 4-hydroxynomenol (4-HNE). Measurements of lipid peroxidation are considered to be of great importance in environmental risk assessment17. Additionally, fish constitute an important link in food chain and their contamination by produced water is capable of disrupting aquatic ecosystems, therefore it is imperative to investigate the toxic effects of produced water on fish, because it is the major waste water discharged from Nigeria oil and gas industry.
MATERIALS AND METHODS
Study area: The study was carried out at Bioassay Laboratory of Nigerian Institute for Oceanography and marine Research, Lagos from 07/05/2018 to 28/09/2018.
Bioassay Containers: Two litre capacity of transparent rectangular plastic tanks was used for the acute toxicity bioassay experiments of Produced formation water against the brackish water Tilapia guineensis. While ten litre capacity of transparent cylindrical plastic tanks was used for sub-lethal toxicity bioassays of produced formation water against Tilapia guineensis18.
Test Media Preparation for Acute Toxicity Bioassay: Test concentrations of produced formation water were prepared using filtered habitat water as a diluent in 2 litre capacity tanks19, followed by a preliminary range finding test.
Range Finding Tests: Tests for Range finding were carried out to establish a preliminary working range by obtaining the least concentration that gives no effect and the minimum concentration that give 100% mortality. During the range finding test of produced formation water acting against Tilapia guineensis, 700 ml test concentration of produce water + 300 ml of habitat water gave 100% mortality while 30 ml test concentration of produce water + 970 ml of habitat water gave 0% mortality within 96 hours hence the following test concentrations were used for the bioassay: 0 ml/L (control), 100 ml/L, 300 ml/L, 400 ml/L, 500 ml/L, 700 ml/L.
Definitive Tests: Active Tilapia guineensis fry after 14 days of acclimatization were randomly picked and transferred to the bioassay tank already holding different treated test media. For the series of bioassay, 10 fishes were exposed per treatment including untreated control in two replicates (5 fishes per replicate) per treatment. The test organisms were exposed to graded series of test concentrations. Mortality assessments were carried out once every 24 hours over a 96 hour period20.
Assessment of Quantal Response (Mortality): Mortalities were recorded cumulatively throughout the test period at 24, 48, 72, and 96 hours. Dead fish were removed from the bioassay tank immediately to prevent contamination. Quantal response was assessed by taking mortality counts at different intervals of 24 hours of commencement of bioassay over a 96 hour period. Tilapia guineensis were taken to be dead when there was no observed body movement and the organism failed to respond by moving away from initial position when prodded with a glass rod or they were observed to be floating motionless on their backs and not swimming21.
Dose-Response Probit Analysis: The toxicity of the test chemicals was evaluated by the use of quantal response, from which the relationships between concentration and percentage effect (response) was defined. In the 96 hour test, the 24, 48, 72, and 96 hour LC50 and their 95% confidence interval were computed to probit regression analysis model22. The probit analysis was done using the Statistical Packages for Social Sciences (SPSS) software, version 20. The toxicity factors showing the relative toxicity of the test chemicals to the given organism was also computed. T.F = Toxicity for relative potency measurement i.e. ratio of 96 hr-LC50 of the most toxic test chemical to the 96 hr- LC50 of the least toxic chemical.
Sub-lethal Toxicity Studies: Sub-lethal toxicity tests were carried out on the fingerlings of Tilapia guineensis to evaluate the effects of sub-lethal concentrations of produced water on Tilapia guineensis, test media were set up to expose them to 1/10th, 1/100th, and 1/1000th, of the predetermined 96 hour LC50 value of the produced water corresponding to 31.08 ml, 3.11 ml and 0.31 ml over a period of 28 days.
Biochemical Studies: Three fishes were collected from each of the test concentration media and control media on the 14th and 28th day of the experiment to assess changes in liver antioxidant activity, the level of lipid peroxidation products as well as to determine liver function enzyme activity in the whole samples against the control. The fishes were dissected using sterile dissecting kit. The liver was carefully harvested using a pair of scissors and forceps, placed in labelled air-tight specimen bottles and preserved in the freezer for biochemical analysis. The activities of antioxidant enzymes; CAT, SOD and GSH and antioxidant molecule GSH as well as the lipid peroxidation product (MDA) were assessed23.
Homogenizing the Liver Samples: The post mitochondria fraction of the liver of each fish was prepared according to Habbu et al.24. The Liver were washed in an iced cold 1.15% potassium chloride solution, blotted and then homogenized with 0.1 M phosphate buffer (pH 7.2), before putting each of the organs into the mortar; laboratory sand was added to it (acid washed sand) and it was blended together in the mortar with a pestle. The resulting homogenate was centrifuged at a speed of 2500 rpm for 15 minutes after which it was removed from the centrifuge. The supernatant was decanted and antioxidant enzyme activities were determined spectrophotometrically using UV-Visible spectrophotometer. The following antioxidant enzymes activities were determined: Superoxide Dismutase activity, Catalase activity, Reduced Glutathione (GSH) content, Urea, Aspartate aminotransferase (AST), Alanine transaminase (ALT), Lipid peroxidation and Glutathione –S- transferase activity.
Statistical Analysis: Data collected from biochemical analysis were presented using the descriptive statistical methods of the means with standard deviations and components bar charts with error bars. Inferential statistical technique of the one-way ANOVA was used to test the significance of variation and differences in biochemical parameters (SOD, GST, GSH, CAT and MDA) within and between sub-lethal test concentrations and control. Where significant differences (p<0.05) do occur from the ANOVA, the Fishers LSD multivariate comparison was used to detect the source of difference. The statistical analyses were done using the Statistical Packages for Social Sciences (SPSS) software, version 22.
RESULTS
Acute Toxicity Tests of Produce Water against Tilapia guineensis: Table 1 shows the result of the acute toxicity test of produced water against Tilapia guineensis. The result revealed that the LC50 values for produced water for 24 hours, 48 hours, 72 hours and 96 hours were 637.16 ml/l, 536.62 ml/l, 364.23 ml/l, 223. 10 ml.l respectively.
Table 1: Acute Toxicity Profile of Produced Formation Water effects on Tilapia guineensis |
||||
| Treatments | LC50 ml/l | 95% Confidence limit | Probit Equation | Toxicity Factor |
| 24 hours | 637.16 | 504.21 – 609.91 | Y= 5X -14.6 | 1.85 |
| 48 hours | 536.62 | 392.34 – 511.83 | Y= 4.8X -12.8 | 1.57 |
| 72 hours | 364.23 | 286.54 – 505.23 | Y= 4.3X -10.6 | 1.23 |
| 96 hours | 223.10 | 244.20 – 553.11 | Y= 4.3X -10.6 | 1.10 |
The result of the effects of sub-lethal concentrations of produced water on the antioxidant defence molecule of reduced glutathione level in the liver of Tilapia guineensis is shown in Fig. 1. The Reduced Glutathione level (9 mmol l−1) did not significantly increased (p> 0.05) at the end of 28 days treatment when compared to day 14, signifying that the GSH function of normal maintenance of metabolic activity has been affected and consequently decreased from the normal value of 10 mmol l−1.
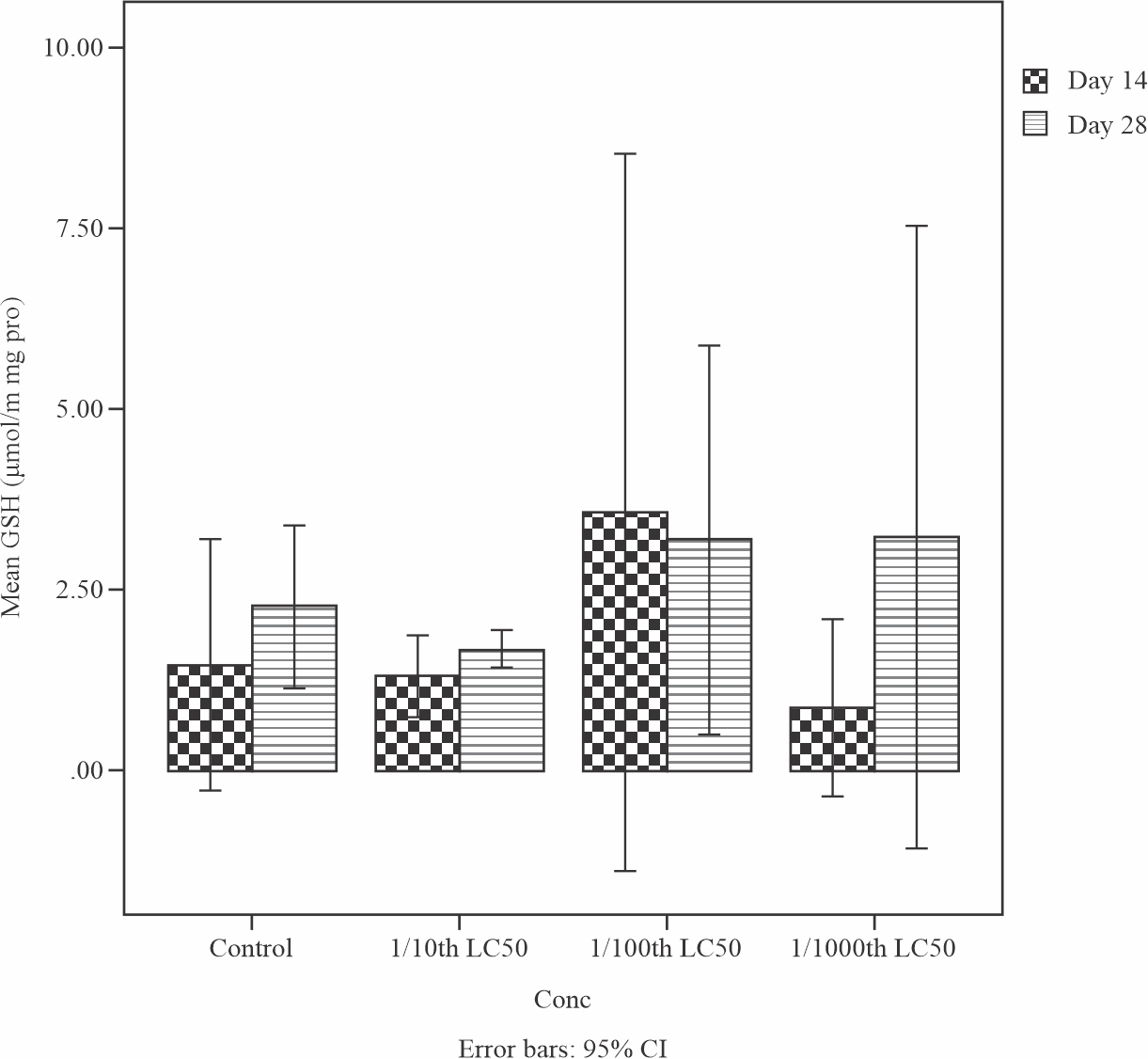 |
Fig. 1: Mean levels of reduced glutathione (GSH) in the liver of Tilapia guineensis exposed to Produced formation water after 14 and 28 days |
Figure 2 illustrate the result of the effects of sub-lethal concentrations of produced water on the superoxide dismutase (SOD) level in the liver of Tilapia guineensis. SOD level increased but not significant (p>0.05) at the end of 28 days treatment when compared to day 14. This observation shows that the cellular homeostasis function of superoxide dismutase (SOD) of Tilapia guineensis exposed to Produced formation water have been impaired.
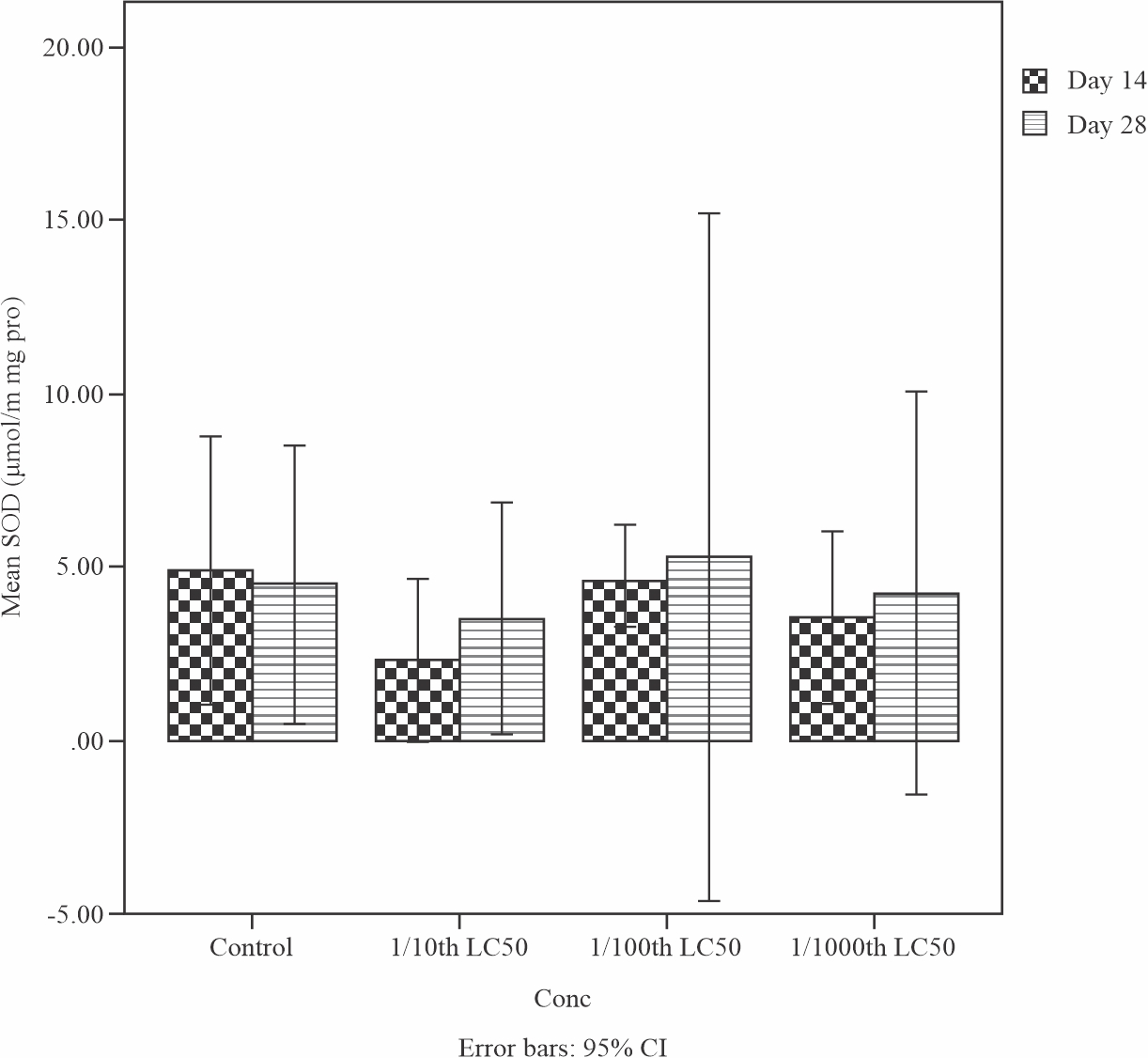 |
Fig. 2: Mean levels of Superoxide dismutase (SOD) in the liver of Tilapia guineensis after 14 and 28-days exposure to produced water |
Moreover, result of the effects of sub-lethal concentrations of produced water on the Catalase (CAT) level in the liver of Tilapia guineensis is shown in Fig. 3. CAT level decreases but was not significant (p>0.05) at the end of 28 days treatment when compared to day 14. Empirically, the decrease of this enzyme (Catalase) signifies that their function had been altered and consequently can allow hydrogen peroxide to build up to toxic level in the liver of Tilapia guineensis exposed to Produced water.
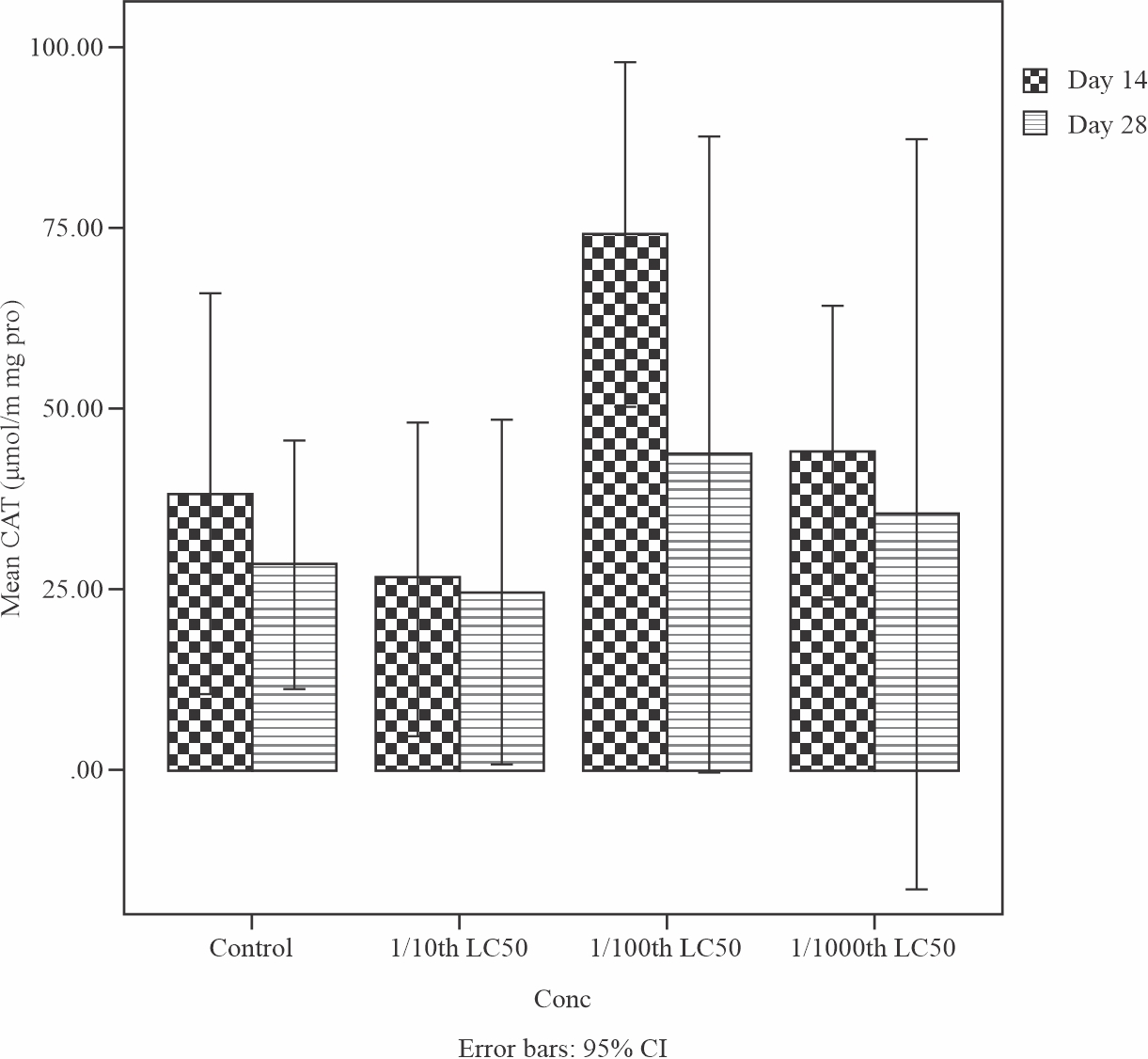 |
Fig. 3: Mean levels of Catalase (CAT) in the liver of Tilapia guineensis after 14 and 28 days exposure to produced water |
The effects of sub-lethal concentrations of produced water on the Glutathione -s- transferase (GST) level in the liver of Tilapia guineensis is shown in Fig. 4. GST level increased but was not significant (p>0.05) at the end of 28 days treatment when compared to day 14. This enzymes helps to neutralize the active electrophilic sites by making toxic chemical to be more water soluble in order to speed up their excretion processes. GST levels were insignificantly (0.866) increased by Day 28; comparison between control and all treatments showed an insignificant difference (0.647, 0.729 and 0.882 respectively) on Day 28.
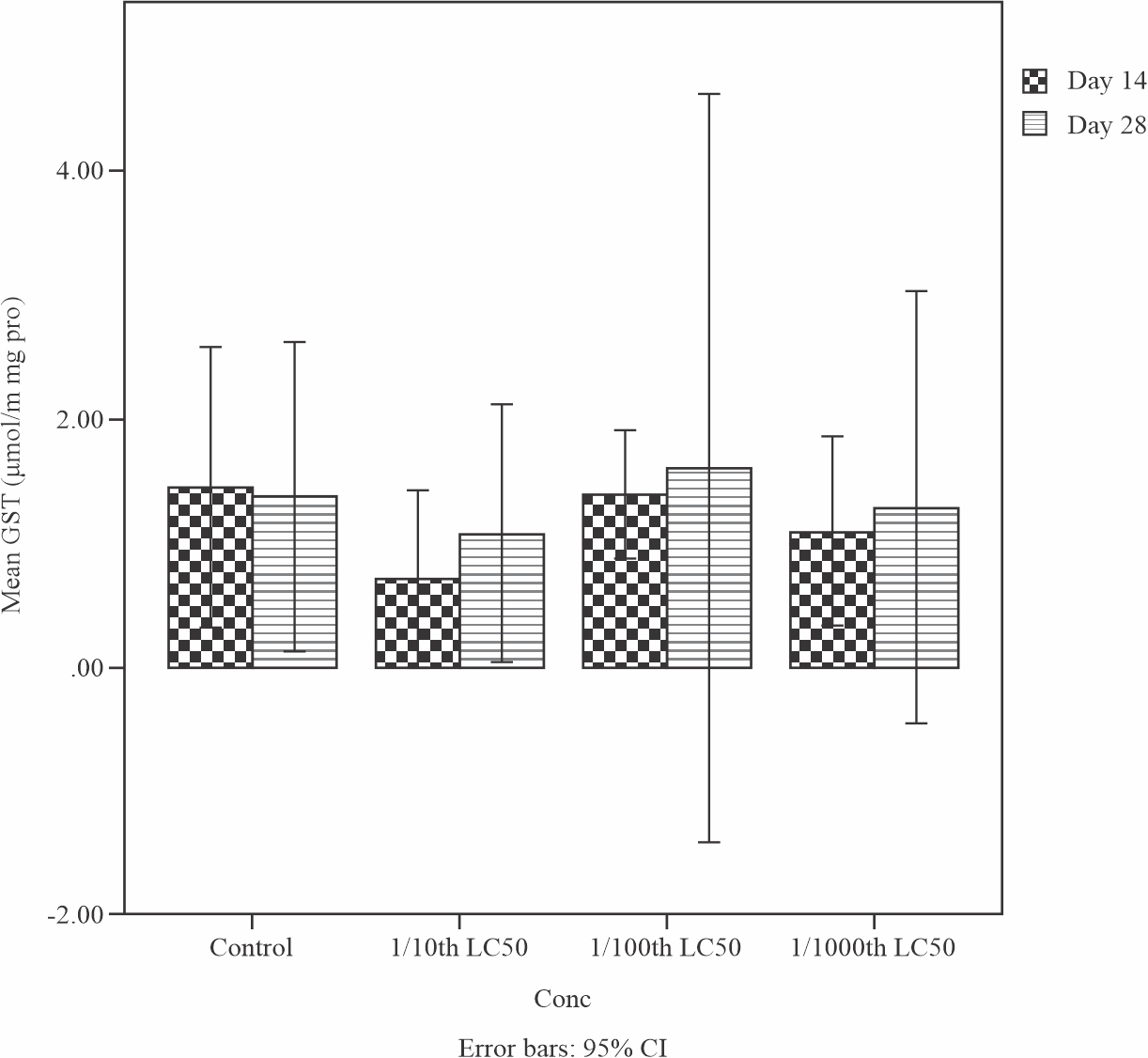 |
Fig. 4: Mean levels of Glutathione-s-transferase (GST) in the liver of Tilapia guineensis exposed to produced water after 14 and 28 days |
Effects of sub-lethal concentrations of produced water on the lipid peroxidation product or Maliondealdehyde (MDA) level in the liver of Tilapia guineensis is shown in Fig. 5. MDA level decreased significantly (p<0.05) at the end of 28 days treatment when compared to day 14. This observation reveals that Maliondealdehyde (MDA) levels reduced insignificantly (0.129) by Day 28. The low level of lipid peroxidation in the liver of Tilapia guineensis as indicated by reduced MDA production suggests that the produced water did not cause significant free radical induced oxidative cell injury.
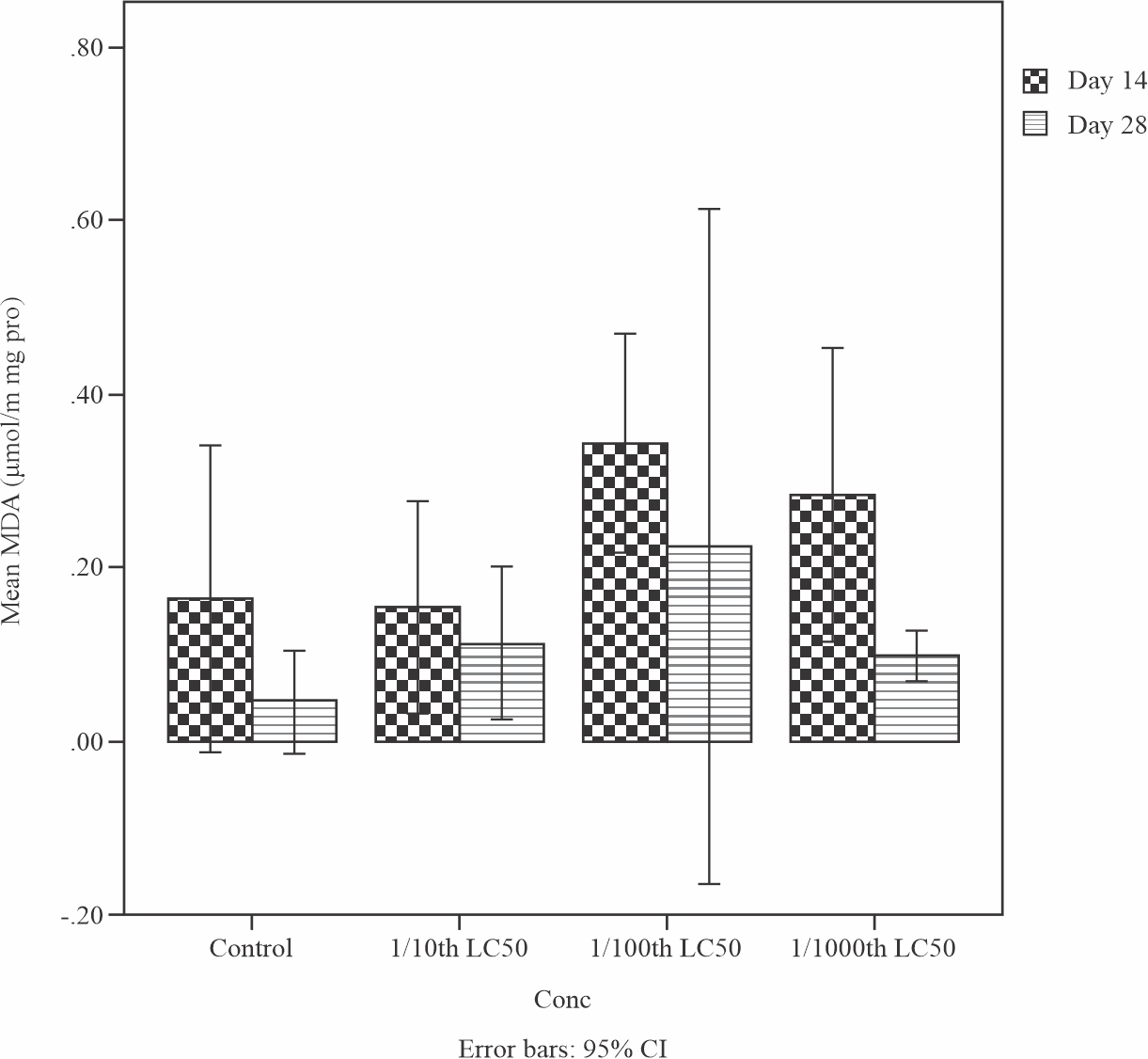 |
Fig. 5: Mean levels of Lipid peroxidation product Maliondealdehyde (MDA) in the liver of Tilapia guineensis after 14 and 28-days exposure to produced water |
Figure 6: shows the result of the effects of sub-lethal concentrations of produced water on the Urea level in the liver of Tilapia guineensis. The urea level was 18.6mmol/L higher than the normal range of the control which was observed at 5.8 mmol/L. This increase in the urea level is an indication that the fish liver have been damaged after exposure on Produced water.
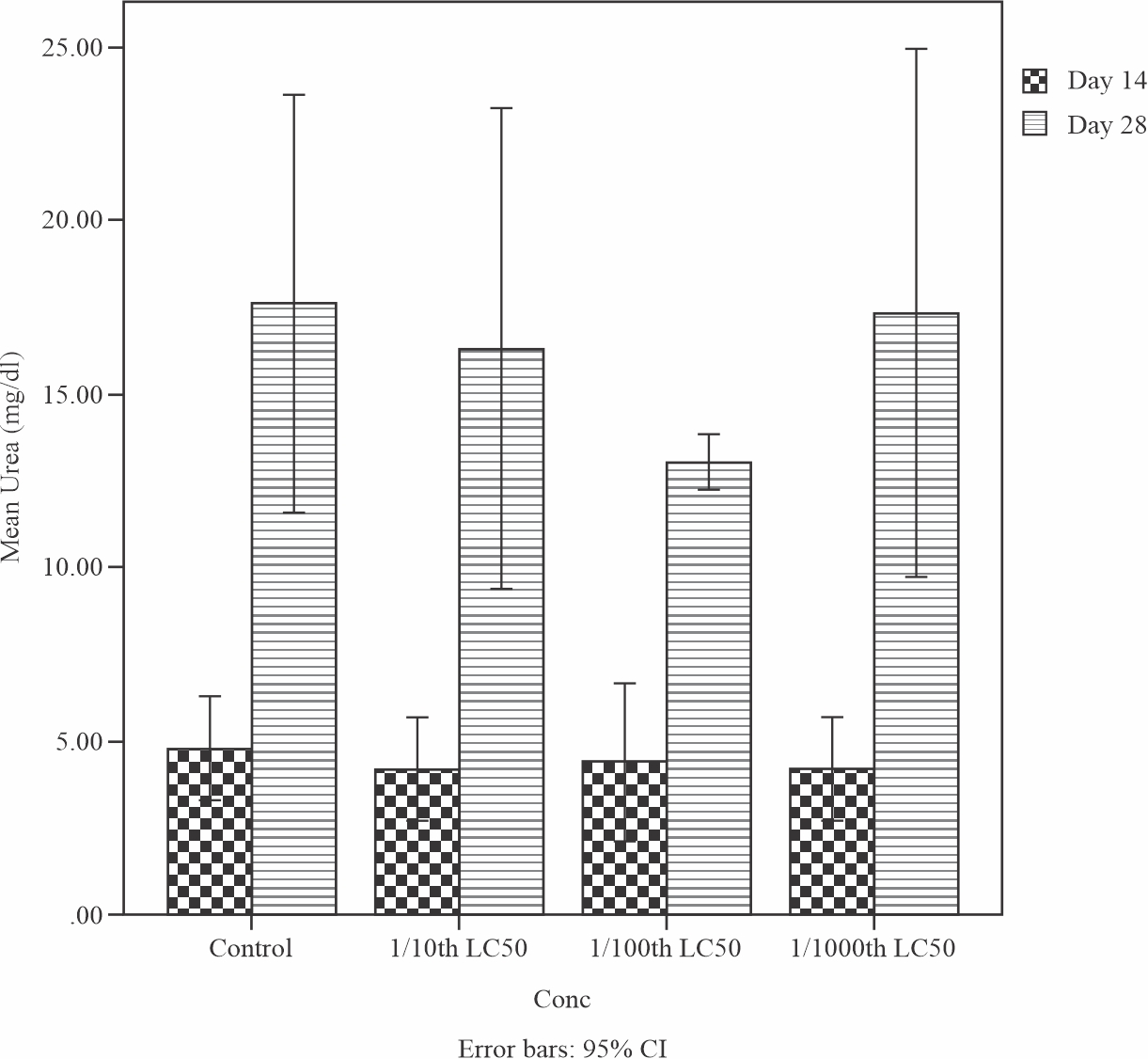 |
Fig. 6: Mean levels of Urea in the liver of Tilapia guineensis after 14 and 28-days exposure to produced water |
Effects of sub-lethal concentrations of produced water on the Alanine transaminase (ALT) level in the liver of Tilapia guineensis is shown in Fig. 7. An increase level of ALT was observed with a value of 55 U/L above the normal range of 46 U/L from the control fish indicating that the fish liver is damaged as a result of oxidative stress effect from the Produced water.
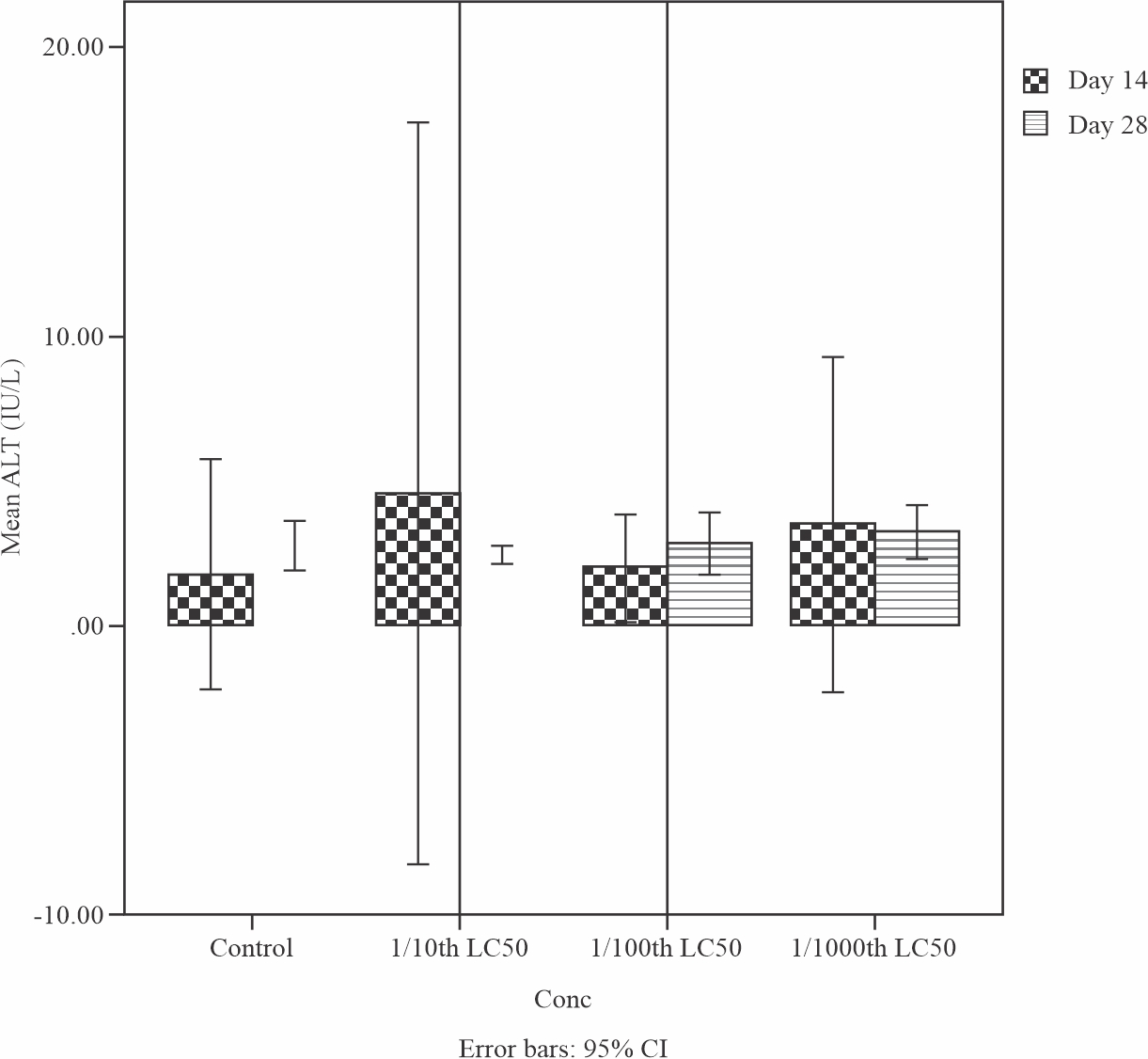 |
Fig. 7: Mean levels of Aspartate aminotransferase (AST) in the liver of Tilapia guineensis after 14 and 28-days exposure to produced water |
Effects of sub-lethal concentrations of produced water on Aspartate aminotransferase level in the liver of Tilapia guineensis is shown in Fig. 8. A decreased level of AST (4 IU/L) was observed from a normal range 5 – 40 IU/L and it’s an indication of liver damage meaning that the Produced water exert it toxicity effect on the fish liver.
 |
Fig. 8: Mean levels of Aspartate aminotransferase (AST) in the liver of Tilapia guineensis after 14 and 28-days exposure to produced water |
DISCUSSION
The biochemical effects such as lipid peroxidation, antioxidants defence molecule and liver function enzymes assays as well as acute toxic effects of produced water on Tilapia guineensis was evaluated in this study. The toxic effects of produced water on Tilapia guineensis was observed to vary significantly depending on the dose-response time of Tilapia guineensis to acute and sub-lethal concentrations of produced formation water.
The 96-hour LC50 values for Tilapia guineensis was 223.10 ml/l on produced water, signifying low acute toxicity, and it is in agreement with the study of Nkwelang et al.25 whose report shows the acute toxicological effects of produced formation water on Tilapia guineensis. However, actual effects of acute toxicity of produced water on any living tissue depend on the concentrations of the toxicant that exist over the exposure period of time found in the environment26. Thus, produced water was found to be acutely toxic to Tilapia guineensis at high concentrations with increasing exposure periods from this study.
Changes in the biochemical profile of Tilapia guineensis exposed to different concentrations of produced water was recorded from this study and it’s an essential sub-lethal effects of long term exposure to small concentrations of toxicants. Nonetheless, such manifestations of biochemical effects include: formation of oxidative stress enzymes, alterations of antioxidant defence activity and inhibition of protein synthesis27. There was depression in the activities of CAT, SOD and GSH which indicates some level of oxidative stress acting in the liver of Tilapia guineensis exposed to Produced water. The low level of Maliondealdehyde in the highest concentration of the produced water however did not correlate with the much higher levels of Maliondealdehyde observed in the lower concentrations and exposure period when compared to the control. Reduced glutathione is the main intracellular antioxidant and has a key role in the detoxification process of pollutants, not only as a substrate of antioxidant enzymes, but as a direct reducing agent and a nucleophile able to block the toxicity of organic chemicals with thio affinity by covalent bonding28. GSH level of Tilapia guineensis from this study was observed to change as a result of exposure to produced water which collaborate with the finding of Poljsak and Fink29, who reported that a variety of environmental pollutants are known to change the GSH level in aquatic organisms, including petroleum based effluents. The cellular defence system against organic pollutant toxicity consist of induction of SOD and CAT. SOD catalyzes scavenging of superoxide anion radicals (O2-) to hydrogen peroxide H2O230 and CAT detoxifies H2O2 to water and oxygen. This study revealed that the SOD and CAT activities in the liver of Tilapia guineensis exposed to sub-lethal concentrations of produced water created different responses. SOD levels did not increase significantly by day 28 of exposure of the Tilapia guineensis to the produced water. Comparison between control and all treatments showed an insignificant difference on day 28. CAT levels did not change significantly as well by day 28 of exposure and specifically, comparison between control and all treatments showed no significant difference on day 28 but there was slight depression of CAT activities in those exposed to the highest concentrations. Induction of SOD and CAT activities is revealing that the oxidative stress response is still active for the lower concentrations of exposure unlike in the highest concentrations where there are signs of inhibition. Thus exposures to high concentrations of produced water as in the case of accidental spills of large quantities may result in marked inhibition of these enzymes.
Glutathione -s- transferase levels did not increase significantly by day 28 and comparison between control and all the treatments showed no significant difference on day 28 and it is in agreement with reports gotten from Atlantic cod exposed to North Sea oil and alkyl phenols for 15 days31 and Prochilodus lineatus exposed to diesel oil for 15 days but contradicts with results gotten from study of Clarias gariepinus exposed to Polycyclic Aromatic Hydrocarbons32. Perhaps, this could be due to the differences in fish species or due to the nature of the test compounds.
This study also discovered that liver function enzyme activities in the liver of Tilapia guineensis exposed to sub-lethal concentrations of produced water produced different responses as Urea and GST enzyme levels increases, but was not significant by day 28 and comparison between control and all the treatments showed no significant difference for AST; while 1/100 treatment showed a significant difference for Urea on day 28. The enzyme ALT decreased but was not significant by day 28 after comparison between control and all treatments.
CONCLUSION
The use of Fish Biomarkers in this study have been found useful in diagnosing environmental contamination and assessment of the effects of toxicant on living organisms. Fishes are very sensitive indicators of petroleum based effluent toxicity, hence the reason they are being employed in ecotoxicological studies both in the laboratory and in the field. Moreso, the results of this assessment showed that the dose-time dependent acute toxicity response and the concentration of the Produced water has a significant effect on the biochemical processes and tissues of Tilapia guineensis such as the alterations of GSH, SOD, GST, CAT, MDA, Urea, AST and ALT activity. Moreover, these biochemical parameters are usually the first detectable and quantifiable responses to environmental changes.
SIGNIFICANCE STATEMENT
This study discovered that changes in the liver of Tilapia guineensis that was exposed to petroleum effluents can be used to evaluate the impact of these effluents. Therefore, the assessed parameters in the fish could serve as a sentinel or a good battery of biomarkers for swift detection of pollution during bio-monitoring programmes in the oil industry. This study will also help researchers to explore the influence of effluents on the biochemical pathways and enzymatic functions of aquatic fauna in the gulf of guinea and other places where Produced water are discharged. Thus a new frontier on environmental risk assessment maybe arrived at.
REFERENCES
- Ackerman, P.A., R.B. Forsyth, C.F. Mazur and G.K. Iwama, 2000. Stress hormones and the cellular stress response in salmonids. Fish Physiol. Biochem., 23: 327-336.
- Adams, S.M., 2002. Biological indicators of aquatic ecosystem stress. Am. Fish. Soc., 3: 104-112.
- Ahmad, I., T. Hamid, M. Fatima, H.S. Chand, S.K. Jain, M. Athar and S. Raisuddin, 2000. Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim. Biophys. Acta, 1523: 37-48.
- Armstrong, R.N., 1997. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol., 10: 2-18.
- Arukwe, A., S.W. Kullman and D.E. Hinton, 2001. Differential biomarker gene and protein expressions in nonylphenol and estradiol-17β treated juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C, 129: 1-10.
- Arukwe, A., T. Nordtug, T.M. Kortner, A.S. Mortensen and O.G. Brakstad, 2008. Modulation of steroidogenesis and xenobiotic biotransformation responses in zebrafish (Danio rerio) exposed to water-soluble fraction of crude oil. Environ. Res., 107: 362-370.
- Banni, M., Z. Bouraoui, J. Ghedira, C. Clerandeau, H. Guerbej, J.F. Narbonne and H. Boussetta, 2009. Acute effects of benzo[a]pyrene on liver phase I and II enzymes, and DNA damage on sea bream Sparus aurata. Fish Physiol. Biochem., 35: 293-299.
- Stadtman, E.R. and B.S. Berlett, 1997. Reactive oxygen-mediated protein oxidation in aging and disease. Chem. Res. Toxicol., 10: 485-494.
- Ergene, S., T. Cavas, A. Celik, N. Koleli, F. Kaya and A. Karahan, 2007. Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): Genotoxic in relation to water pollution. Ecotoxicology, 16: 385-391.
- Barton, B.A., J.D. Morgan and M.M. Vijayan, 2002. Physiological and Condition-Related Indicators of Environmental Stress in Fish. In: Biological Indicators of Aquatic Ecosystem Stress, Adams, S.M. (Eds.). Am. Fish. Soc., Bethesda, Maryland, pp: 111-148.
- Binelli, A., D. Cogni, M. Parolini, C. Riva and A. Provini, 2009. Cytotoxic and genotoxic effects of in vitro exposure to Triclosan and Trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp. Biochem. Physiol. C: Toxicol. Pharmacol., 150: 50-56.
- Capkin, E., S. Birincioglu and I. Altinok, 2009. Histopathological changes in rainbow trout (Oncorhynchus mykiss) after exposure to sublethal composite nitrogen fertilizers. Ecotoxicol. Environ. Saf., 72: 1999-2004.
- Chapman, P.M., A. Fairbrother and D. Brown, 1998. A critical evaluation of safety (uncertainty) factors for ecological risk assessment. Environ. Toxicol. Chem., 17: 99-108.
- Chovanec, A., R. Hofer and F. Schiemer, 2003. Chapter 18 Fish as Bioindicators. In: Trace Metals and other Contaminants in the Environment, Mushak, P. (Ed.)., Elsevier, New York, pp: 639-676.
- Correia, A.D., R. Gonçalves, M. Scholze, M. Ferreira and M.A.R. Henriques, 2007. Biochemical and behavioral responses in gilthead seabream (Sparus aurata) to phenanthrene. J. Exp. Mar. Biol. Ecol., 347: 109-122.
- Danzo, B.J., 1997. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ. Health Persp., 105: 294-301.
- Van der Oost, R., J. Beyer and N.P.E. Vermeulen, 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol., 13: 57-149.
- ASTM, 1993. Standard Guide for Conducting Acute Toxicity Tests with Fishes, Macroinvertebrates and Amphibians. Annual Book of ASTM Standards. Am. Soc. Test. Mater., Philadelphia, PA, USA, pp: 88–729.
- Ebrahimpour, M., H. Alipour and S. Rakhshah, 2010. Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicol. Ind. Health, 26: 361-365.
- Di Giulio, R.T. and D.E. Hinton, 2008. The Toxicology of Fishes. 1st Edn., CRC Press, Boca Raton.
- CouIllard, C.M., K. Lee, B. Legare and T.L. King, 2005. Effect of dispersant on the composition of the water-accommodated fraction of crude oil and its toxicity to larval marine fish. Environ. Toxicol. Chem., 24: 1496-1504.
- Finney, D.J., 1971. Probit Analysis. 3rd Edn., Cambridge University Press, London, UK., pp: 76-80.
- Gutteridge, J.M., 1995. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem., 41: 1819-1828.
- Habbu, P.V., R.A. Shastry, K.M. Mahadevan, J. Hanumanthachar and S.K. Das, 2008. Hepatoprotective and antioxidant effects of Argyreia speciosa in rats. Afr. J. Tradit. Complement. Altern. Med., 5: 158-164.
- Nkwelang, G., G.E. Nkeng, H.L.K. Fouamno and S.P. Antai, 2009. Effect of crude oil effluent (produced water) on brackish water fish and microbial growth in aquarium environment. Pac. J. Sci. Technol., 10: 619-625.
- Opete, O.S.E., L.C. Osuji and A.I. Hart, 2019. Acute toxicity of Tilapia guineensis fingerlings exposed to treated produced water from Niger Delta region of Nigeria. Int. J. Res. Stud. Biosci., 7: 8-21.
- Simonato, J.D., C.L.B. Guedes and C.B.R. Martinez, 2008. Biochemical, physiological and histological changes in the neotropical fish Prochilodus lineatus exposed to diesel oil. Ecotoxicol. Environ. Saf., 69: 112-120.
- Vasseur, P. and C. Leguille, 2004. Defense systems of benthic invertebrates in response to environmental stressors. Environ. Toxicol., 19: 433-436.
- Poljsak, B. and R. Fink, 2014. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxidative Med. Cell. Longevity., 1: 1-22.
- Nordberg, J. and E.S.J. Arner, 2001. Reactive oxygen species, antioxidants and the mammalian thioredoxin system. Free Radic. Biol. Med., 31: 1287-1312.
- Sturve, J., L. Hasselberg, H. Fälth, M. Celander and L. Förlin, 2006. Effects of North Sea oil and alkylphenols on biomarker responses in juvenile atlantic cod (Gadus morhua). Aquat. Toxicol., 78: S73-S78.
- Otitoloju, A. and O. Olagoke, 2011. Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. Environ. Monit. Assess., 182: 205-213.
How to Cite this paper?
APA-7 Style
Chidinma,
P., ,
W., Chinedu,
O.F. (2021). Biochemical and Toxicological Evaluation on Tilapia guineensis Exposed to Produced Formation Water. Asian Journal of Emerging Research, 3(1), 70-74. https://doi.org/10.3923/ajerpk.2021.70.74
ACS Style
Chidinma,
P.; ,
W.; Chinedu,
O.F. Biochemical and Toxicological Evaluation on Tilapia guineensis Exposed to Produced Formation Water. Asian J. Emerg. Res 2021, 3, 70-74. https://doi.org/10.3923/ajerpk.2021.70.74
AMA Style
Chidinma
P,
W, Chinedu
OF. Biochemical and Toxicological Evaluation on Tilapia guineensis Exposed to Produced Formation Water. Asian Journal of Emerging Research. 2021; 3(1): 70-74. https://doi.org/10.3923/ajerpk.2021.70.74
Chicago/Turabian Style
Chidinma, Peac, Wilfred-Ekprikpo , and Ogbonne Fabian Chinedu.
2021. "Biochemical and Toxicological Evaluation on Tilapia guineensis Exposed to Produced Formation Water" Asian Journal of Emerging Research 3, no. 1: 70-74. https://doi.org/10.3923/ajerpk.2021.70.74

This work is licensed under a Creative Commons Attribution 4.0 International License.




