Inhibition of Prostate Cancer by Plant Extracts Used in the Treatment of Malaria in Burkina Faso
| Received 09 Nov, 2020 |
Accepted 27 Feb, 2021 |
Published 03 Jun, 2021 |
Background and Objectives: Medicinal plants constitute the major source of malaria treatment in a different region of Burkina Faso, the present study was carried out to determine the potentially anti-malaria plants of the region of Bobo Dioulassoand to assess preliminary investigations on the possible pharmacological applications others than anti-malaria properties assessed by traditional health practitioners. Materials and Methods: Open-ended and structured interviews were used during the ethnobotanical survey. Total phenolics and flavonoids content were estimated using spectrophotometric methods. Antioxidant potential was evaluated using anti-radical and reduction methods while the crystal violet staining technique was used for the determination of extracts cytotoxicity on 22RV1 prostate cancer cells. Results: Ethnobotanical survey highlighted five plants from different families as the most used against malaria. Their extracts have shown a significant potential for trapping radicals by the ABTS method. The methanolic extract of T. macroptera has the highest content of phenolic and flavonoids compounds with respectively 59.94±0.15 mg EAG/100 mg of extract and 5, 35±0.05 EQ/100 mg of extract. Methanolic extracts concentration of different plants tested that inhibit 50% of the proliferation of cancer cells 22RV1 proliferation were ranged between 48.34 to 359.1 μg/mL. E. africana was the most active 48.34 μg/mL on tested cell strain. Conclusion: Phytochemical composition and antioxidant potential may justify the use of tested plants in the treatment of malaria. Antiradical potential and reduction capacities of tested samples may explain their toxicity 22RV1 prostate cancer cells.
INTRODUCTION
Malaria is among the tropical diseases with a high incidence. It is estimated to contribute to more than one million deaths per year in sub-Saharan Africa1 where it is among the top five diseases causing death in children2,3. Burkina Faso is one of the ten countries with the highest number of malaria cases and associated deaths (3% of cases and deaths worldwide). Malaria is responsible for 43% of health consultations and 22% of deaths4. It is characterized generally by fever, chills, and flu-like illness5.
During the malaria crisis, oxidative stress is highly involved in the release of the ferrous ion (Fe2+) from haemoglobin and its crystallization into ferric ion (Fe3+) in the form of hemozoin by the parasite; the release of cytokines and other chemical mediators implicated in the amplification of the release of radicals6,7. The current treatment of malaria is based on the use of compounds belonging to the alkaloids molecular class. Unfortunately,the ability to treat and control Plasmodium falciparum infection through chemotherapy has been compromised by the advent and spread of resistance to antimalarial drugs8. Therefore, the need to find New molecular sources capable of eliminating the pathology remains valid. Confronted with malaria since remote times, the populations of endemic areas have been able to exploit medicinal plants for their benefit and numerous studies have now made it possible to shed light on the mechanisms by which the active compounds of these plants act on the plasmodium. Several medicinal plants are commonly used in the treatment of malaria crisis in Burkina Faso, some of them have already been investigated for this purpose and have shown to be richin molecules with a strong antioxidant power such as polyphenols8. The present study purpose was to identify the plants used for the treatment of malaria in the Bobo Dioulasso region and to assess the content of phenolic compounds as well as the antioxidant power of the most promising.
MATERIALS AND METHODS
Ethnobotanic survey area: Ethnobotanical study was realized in Burkina Faso, specifically in Bobo Dioulasso the economical capital of the country. The investigation area is located in the southwestern part of Burkina Faso, at 11°10' North latitude and 4°18' West longitude, approximatively at 360 km from Ouagadougou (political capital), and an altitude of 445 m, it covers about 136.8 km2. The region is mainly inhabited by Bobos, Mossi, Dafing, but other ethnic groups such as Samogo, Fulani and Lobi/Dagara also live there. The local language is Dioulla.
Data collection methods: The survey was carried out during April June 2019. Open-ended and structured interviews were used during the investigation. The interviews were conducted used the local language (Dioulla) in marketplaces and associations facilities. A total of five groups were interviewed and respondents were male and female gender. Their ages varied from 37 to 72. Questionary was focused on the plants used in the formulation of remedies preconized in the treatment of malaria. Plants specimens were then collected in august 2019 in the classified reserve of Dindérésso (30 km southern direction from Bobo Dioulasso) and their identification was done by a botanist of the department of botanic of the University Nazi-Boni (Bobo Dioulasso, Burkina Faso).
Chemical: All solvents were analytical grade. Agilent Cary 60 UV-Vis Spectrophotometer (Thermo Fisher; GENESYS 30, USA) was used in all spectrophotometric measurements. Ascorbic acid, ferric chloride, aluminium chloride, potassium acetate, quercetin, DPPH reagent, Folin-Ciocalteau reagent, gallic acid, sodium carbonate, methanol was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Millipore deionized water was used throughout. Thiazolyl Blue Tetrazolium Bromide (Sigma Aldrich, USA), Dimethyl Sulfoxide (Sigma Aldrich, USA).
Plants collection and preparation: Different plants assayed was collected in the classified forest of Dindérésso (Bobo Dioulasso) in August 2019. Dried before being reduced separately to powder. A sample of 15 g of each plant powder was extracted with methanol (Sigma Aldrich). The crushing plants were macerated for 48 h at 4°C. The resulting mixture was then filtered under vacuum and concentrated under reduced pressure in a rotary evaporator at 40°C. The extracts were further dried using a TELSTAR CRYODOS freeze dryer machine then kept at - 20°C for further analysis. A solution of 30 mg/mL of each plant was prepared in 50% ethanol for the following tests.
Determination of total polyphenol content: The total phenolic content was determined according to the method9. A 10% solution was prepared from the stock solution (30 mg/mL) using 50% ethanol. 100 µl of this solution was mixed with 200 µl of the Folin-Ciocalteau reagent and 2 mL of de-ionized water then incubated at room temperature for 3 min. A sample of 20% aqueous sodium carbonate (w/w, 1 mL) was then added to the mixture. The total polyphenols were determined after 1h of incubation at room temperature. A negative control sample was also prepared using the same procedure. The absorbance of the resulting blue colour was measured at 770 nm. Results were expressed in mg gallic acid equivalents (GAE) per g dry weight of plant material using an equation obtained from a gallic acid calibration curve. The samples were analyzed in triplicate.
Determination of total flavonoids content: The total flavonoids content in the extracts was determined using the aluminium chloride colourimetric method described by9. A known concentration (600 µg/mL) of each extract in methanol was prepared. A 500 µl of the extracts were mixed separately with 0.1 mL of 10% (w/v) aluminium chloride solution, 0.1 mL of 1M potassium acetate solution, 1.5 mL of methanol and 2.8 mL of distilled water. The solutions were thoroughly mixed and incubated at room temperature for 30min. The absorbance of the reaction mixture was measured at 415 nm using a spectrophotometer. The total flavonoids content was determined using a standard curve with quercetin (1 to 25 µg/mL) as the standard. The mean of three readings was used and expressed as mg of quercetin equivalents (QE)/g of the dry extract.
Free-radical scavenging activity: The antioxidant activity of the extracts was assessed based on their ability to scavenge the stable1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical as described previously by9. Various concentrations of plants extract in methanol were prepared (0.15 to 1.5 mg/mL). A methanolic solution of DPPH (3.8 mL, 60 µg/mL) was rapidly mixed with the plant extract (200 µl, 30 mg/mL) in a test tube, with methanol serving as the blank sample and control was also assayed simultaneously. The contents of the tubes were swirled then allowed to stand for 30 min at room temperature in the dark. The absorbance was measured at 517 nm in a spectrophotometer. The scavenging ability of the plant extract was calculated using this equation:
$$ \text { DPPH scavenging activity }(\%)==\frac{\text { Abs. control -Abs sample }}{\text { Abs. control }} \mathrm{X} 100 $$where Abs control is the absorbance of DPPH + methanol; Abs sample is the absorbance of DPPH radical + sample (sample or standard). The total antioxidant activity was expressed as ascorbic acid equivalent/g dry extract. The assay was done in triplicates.
Ferric reducing antioxidant power (FRAP): FRAP assay was performed according to9with minor modifications. 0,1 mL of each extracts (1 mg/mL) was mixed with 0.25 mL of phosphate buffer (0.2 M, pH 6.6) and 0.25 mL of aqueous potassium hexacyanofer rate [K3Fe (CN)6] solution (1%). After 30 min of incubation at 50°C; 0.25 mL of trichloroacetic acid (10%) was added and the mixture was centrifuged at 2000 g for 10 min then the upper floating solution (125 µL) was mixed with water (125 µL) and a freshly prepared FeCl3 solution.
Cytotoxicity assay: 22RV1 were seeded at a density of 5000 cells/well in a 96-well plate and were allowed to attach overnight. Thereafter, cells were treated with various concentrations of the plant's extracts for 24 h. To assess the cytotoxic effect of the five plants extracts, MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay was carried out. Briefly, cells treated with the plant extracts were exposed to tetrazolium MTT at a concentration of 5 mg/mL. Viable active cells reduced yellow MTT salt to insoluble purple formazan, which was dissolved using DMSO. The absorbance of the coloured solution was measured at a wavelength of 570 nm using an Epoch microplate spectrophotometer (BioTek, USA). The obtained absorbance at 570 nm of both control and treated cells were used to calculate the percentage of cell viability. Assuming 100% viability in control cells, the percentage of treated cells viability will be calculated accordingly to the following equation10:
$$ \text { cells }=\frac{\text { Absorbance of treated cells }}{\text { Absorbance of control cells }} \mathrm{X} 100 $$Statistical analysis: All the reactions were performed in triplicate, and data are presented as mean standard deviation. Data were analyzed by one-way analysis of variance followed by Tukey multiple-comparison test. Analysis was done using XLSTAT7.1 software. A p-value less than 0.05 was used as a criterion for statistical significance.
RESULTS AND DISCUSSION
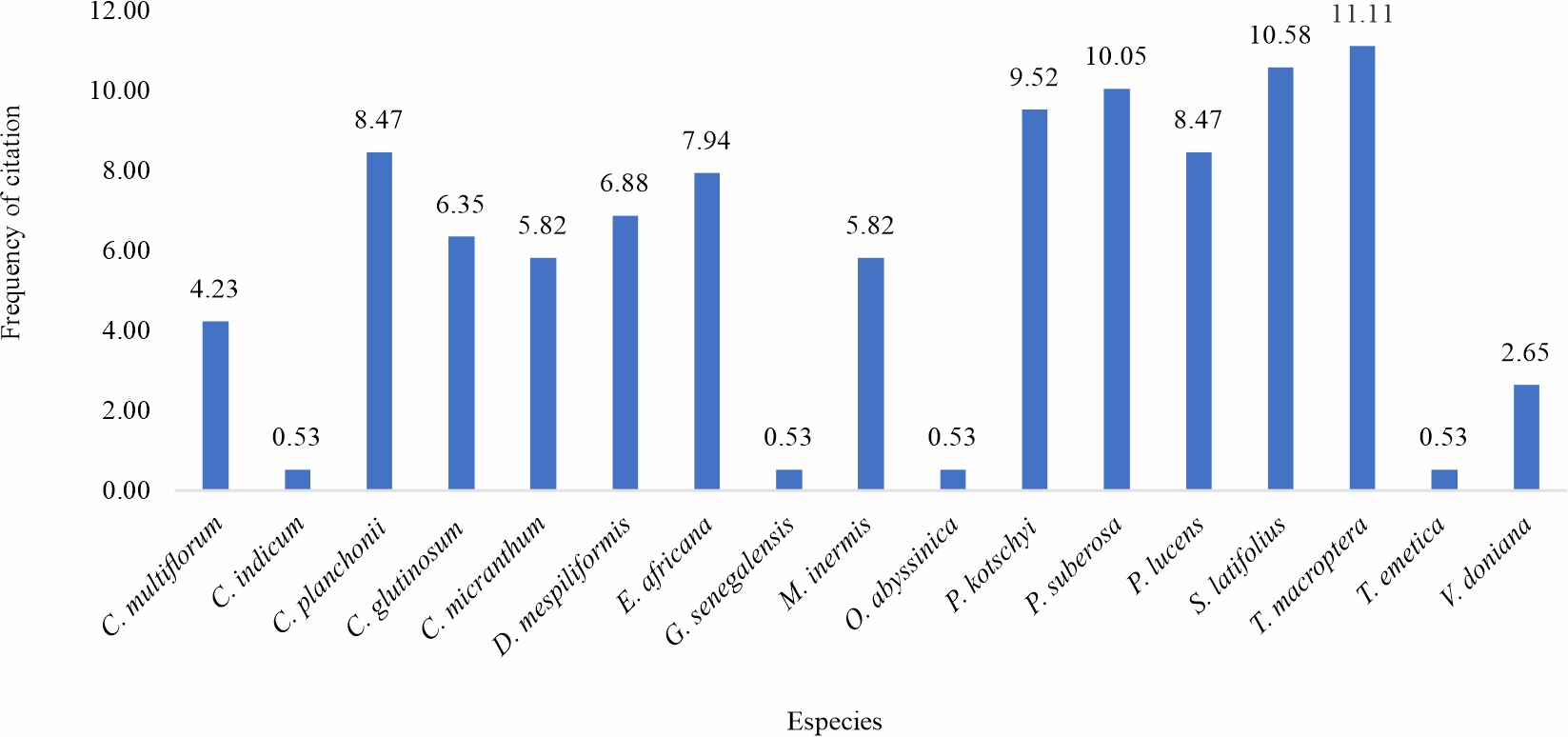
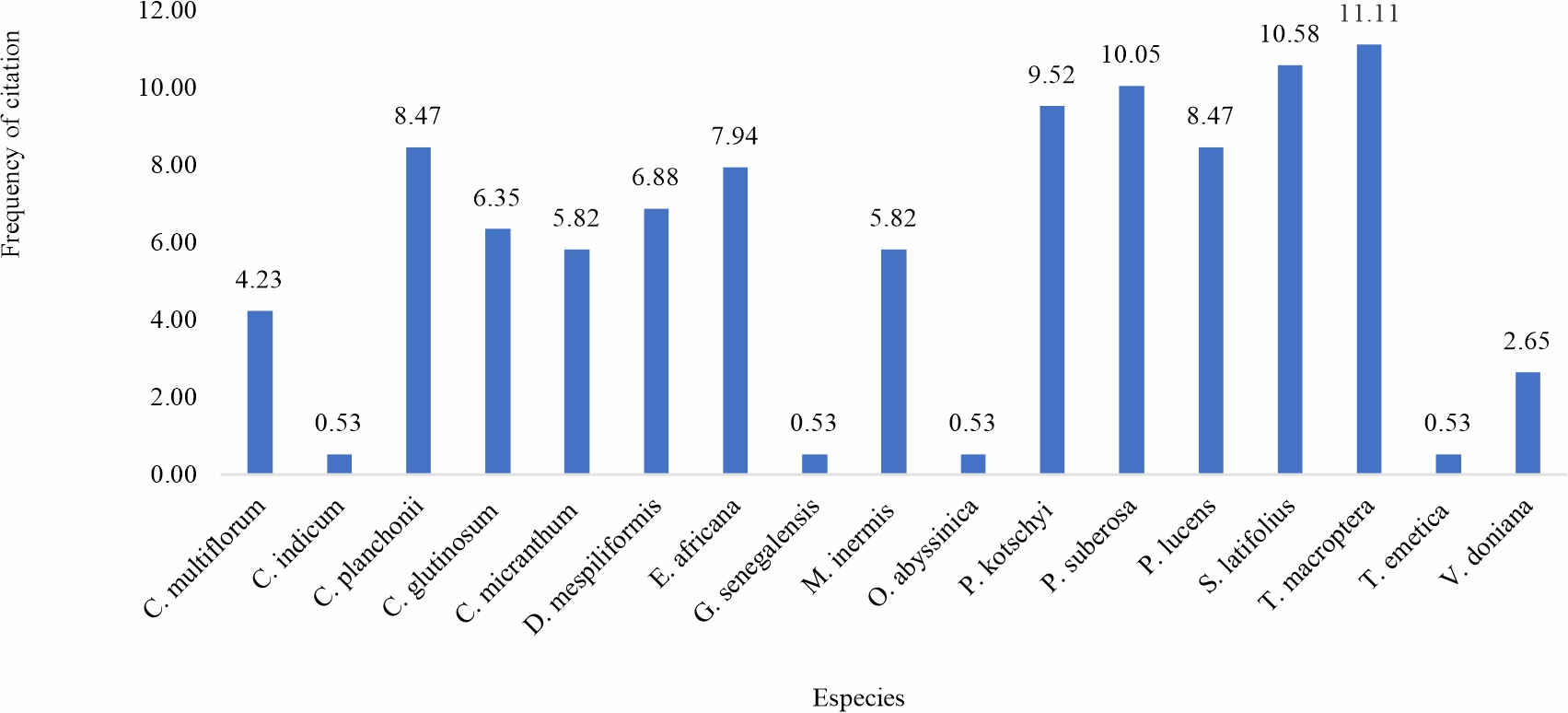
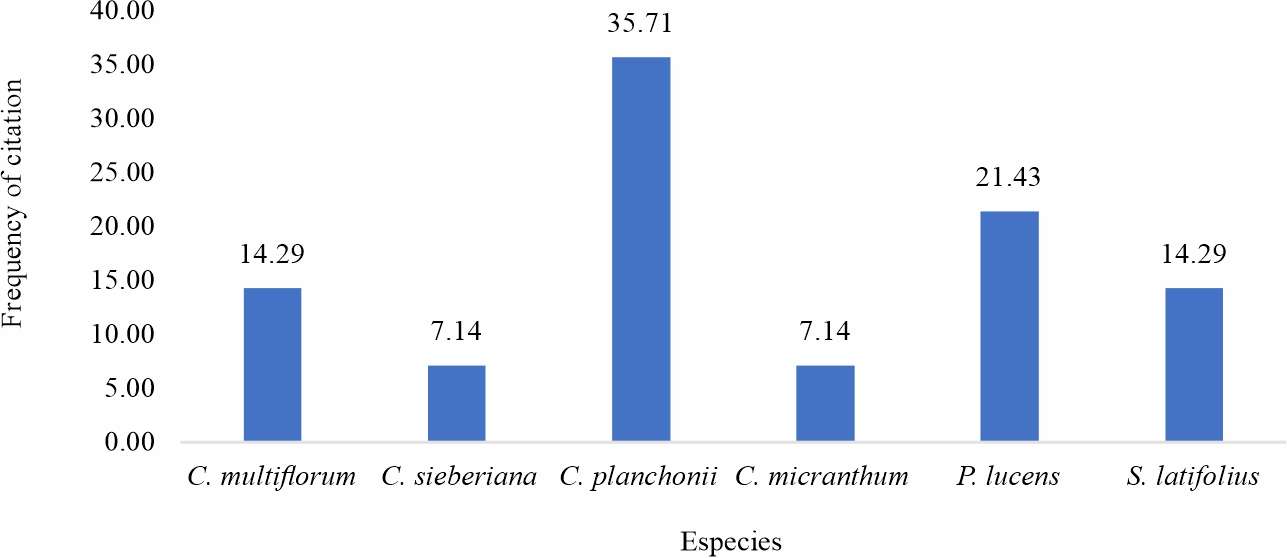
Ethnobotanical survey: Ethnobotanical surveys examined the recipes for medicinal plants used in the treatment of malaria. 53 traditional medicine health practitioners from three groups participated in the survey. A graph is dedicated to each group (Fig. 1-3) and is based on the citation rate of the species. For the first group, 22 traditional practitioners were interviewed on the recipes of plants that each uses for malaria treatment (Fig. 1). A total of 11 plants species were identified in this group. The plants with the best citation rates are Terminalia macroptera (17.72%) >Sarcocephalus latifolius (16.46%) >Cochlospermum planchonii (13.92%) (Fig. 1). Concerning the second group composed of 21 practitioners. 21 health practitioners questioned, the information collected highlights the use of 17 medicinal plants for malaria care, the most cited species of which were Terminalia macroptera with 11.11% of the citations, followed by Sarcocephalus latifolius 10.58% and of Pteleopsissuberosa 10.05% (Fig. 2). The last group surveyed, consisting of 10 persons, the information collected highlights the use of 6 species with C. planchonii (35.71%); Pterocarpus lucens (21.43%). S. latifolius and C. multiflorumeach had 14.29% of citations (Fig. 3). Similar studies in southern Burkina Faso in 2012 on plants used in the treatment of fever-related diseases showed that Sarcocephalus latifolius and Cassia sieberiana were among the most commonly used species11. More recently in Mali12, a study on the development of a traditional improved malaria drug identified Entada africana and Sarcocephalus latifolius as the most commonly used species, with 6.24% and 4.16% respectively. These studies corroborate ours and reinforce the results of our ethnobotanical surveys.
 |
||||||||
Fig. 1: Citation rate of antimalarial medicinal plants preconized by group 2 traditional health practitioners |
 |
||||||||
Fig. 2: Citation rate of antimalarial medicinal plants preconized by group 1 traditional health practitioners |
 |
||||||||
Fig. 3: Citation rate of antimalarial medicinal plants preconized by group 3 traditional health practitioners |
Polyphenol and total flavonoids contents in extracts and fractions of texted samples: The total polyphenol contents and flavonoids contents in the methanolic extracts and the fractions of tested samples expressed in gallic acid equivalent (GAE)/100 mg of extract and quercetin equivalent (QE)/100 mg of the extract are presented in Table 1. T. macroptera was the richness methanolic extract in total phenolic (59.94±0.15 mg (GAE)/100 mg) content and total flavonoids contents, while in DCM fractions phenolic and flavonoids content evaluation revealed that T. macroptera was the richness in phenolic (32.72±1.07 mg (GAE)/100 mg) and S. latifolius, the richness in flavonoids (4.24±0.52mg (QE)/100 mg). Ethyl acetate fractions of C. sieberiana was found to be the one with the highest total phenolic content (61.13±2.72 mg (GAE)/100 mg) and S. latifolius was the richness in flavonoids contents. Previous studies made by13 on C. sieberiana, found 327.16±3.99 mg GAE/100 mg as the content of total polyphenols and 37.270±2.216 mg QE/100 mg as the concentration of flavonoids in roots ethanolic extracts. Their results compared to our results are lower. These difference between the results could be justified by the type of extract used. Indeed14 showed in a comparative study that methanol could extract morepolyphenolic compounds than ethanol one. In addition to alkaloids and their derivatives, many phenolic compounds have been found very promising in the treatment of malaria15. Significant content in phenolic compounds may then justify the use of these species in the treatment of malaria crisis as assumed by the traditional health practitioners questioned.
Antiradical and reduction power of testes medicinal plants: The antiradical and reducing power of different methanolic extracts, DCM and ethyl acetate fractions are presented in Table 2. Through Ferric Reducing antioxidant power evaluation values obtained varied from 410.98±40.10 µmol AAE/g to 5267.59±20.03 µmol AAE/g C. sieberiana and C. planchonii values were statistically the same, while T. macroptera presented the best activity in reducing the ferric ion to ferrous one. Values obtained with dichloromethane and ethyl acetate fractions varied from 121.56±30.06 µmol AAE/g (DCM fraction of S.latifolius) to 1673.99±206.03 µmol AAE/g (ethyl acetate fraction of C. sieberiana) (Table 2). For the DPPH radical inhibition test, the results varied from 197.44±19.18 µmol EAA/g to 788.13±14.75 µmol EAA/g for the methanolic extracts and from 36.65±19.88 µmol EAA/g to 818.76±3.87 µmol EAA/g for the tested fractions. The ethyl acetate fraction of C. sieberiana presented the best result (Table 2). The antioxidant activity by the ABTS method expressed in mmol ascorbic acid equivalent per gram (mmol EAA/g) of extract presented in Table 2 reveals for the methanolic extracts that the extract of T macroptera is more reducing of the ABTS radical with 17452.58±249.82 mmol EAA/g, while the methanolic extract of S. latifolia is the least reducing of this radical (9543.63±231.83 mmol EAA/g). The DCM fraction of E Africana and S latifolia are reducers of the ABTS radical with respective close values of 10120.58±890.11 mmol EAA/g and 10096.54±124.91 mmol EAA/g respectively which are however less than that of T macroptera which exhibits the best activity (12548.55±504.83 mmol EAA/g) for this fraction. As for the ethyl acetate fraction, a comparative study of extracts from five plants reveals that the fraction of C. sieberiana is the most reducing of the ABTS radical while that of S latifolia is the least effective. Of all the extracts tested and fractions, the methanolic extract of T macroptera was found to be the best in terms of reduction of the ABTS radical.
|
||||||||
| Total phenolic content mg (GAE)/100mg | Total flavonoids content mg (QE)/100mg | |||||||
| Methanolic extract | DCM Fractions | AE Fractions | Methanolic extract | DCM Fractions | AE Fractions | |||
| Cassia sieberiana | 50.34±1.11b | 20.99±0.74c | 61.13± 2.72a | 2.96±0.10d | 1.03±0.08d | 0.88±0.12d | ||
| C. planchonii | 22.92±0.57c | 27.42±0.64b | 11.02±0.11d | 2.17±0.21e | 0.98 ±0.1e | 1.22±0.24c | ||
| Entada africana | 11.85±0.45e | 13.78±0.45e | 25.42 ±0.62b | 5.14±0.09b | 2.15±00b | 2.01±0.31b | ||
| S. latifolius | 16.78±0.12d | 18.64±0.43d | 11.14±0.37d | 3.2±0.23c | 4.24±0.52a | 3.02 ±0.16a | ||
| T. macroptera | 59.94±0.15a | 32.72±1.07a | 20.14±0.37c | 5.35±0.05a | 1.43±00c | 1.16 ±0.05c | ||
| Data are Mean±SEM (n=3), DCM: Dichloromethane, EA: Ethyl acetate. Values showing the same letter in the same column are not significantly different (p<0.05) from one to another in the same columns | ||||||||
|
||||||||
| Tests | Extract/fraction | C. sieberiana | C.planchonii | E. africana | S. latifolius | T. macroptera | ||
| FRAP (µmol (AAE)/g) | Methanolic extract | 1939.18±55.80 | 1939.40±10.03 | 1105.62±20.06 | 410.98±40.10 | 5267.59±20.03 | ||
| DCM fraction | 492.03±43.70 | 625.18±34.72 | 387.85±10.03 | 121.56±30.06 | 1036.15±36.14 | |||
| A.E fraction | 1673.99±206.03 | NT | 1001.42±20.06 | 966.69±40.10 | 1013.00±20.06 | |||
| DPPH (µmol (AAE)/g) | Methanolic extract | 777.97±10.33 | 709.82±7.67 | 735.38±11.14 | 197.44±19.18 | 788.13±14.75 | ||
| DCM fraction | 262.98±4.43 | 505.57±10.22 | 36.65±19.88 | 148.10±2.56 | 634.92±5.90 | |||
| A.E fraction | 818.76±3.87 | NT | 603.45±8.94 | 371.10±5.32 | 640.80±2.56 | |||
| ABTS (µmol (AAE)/g) | Methanolic extract | 13437.82±83.11 | 13101.46±83.27 | 10745.60±144.24 | 9543.63±231.83 | 17452.58±249.82 | ||
| DCM fraction | 11034.07±472.91 | 10793.68±41.64 | 10120.58±890.11 | 10096.54±124.91 | 12548.55±504.83 | |||
| A.E fraction | 14255.35±166.55 | NT | 9976.34±83.27 | 7235.85±83.27 | 9062.84±434.71 | |||
Prostate cancer cells 22RV1 proliferation inhibition Activities: Different concentrations of S. latifolius,T. macroptera; E. africana; C. planchonii methanolic extracts were tested to determine their cytotoxicity on prostate cancer cells 22RV1 and the results are presented respectively in Fig. 4. Nine concentrations of extracts from each plant species, ranging from 0 to 500 µg/mL, were tested on the prostate cancer cell line. The first concentration was control (0 µg/mL). Five graphs were obtained: Fig. 4a (tested concentrations range from 296.9 to 434.3), Fig. 4b (tested concentrations range from 210.3 to 279.1), Fig. 4c (tested concentrations range from 39.33 to 67.20), Fig. 4d (tested concentrations range from 39.33 to 67.20) and Fig. 4e (tested concentrations range from 111.2 to 160.8). Values expressed as the concentration of extract that inhibits the 50% of the proliferation of cancer cells tested (IC50) were ranged from 48.34 µg/mL to 359.1 µg/mL. E. africana were found to be the most toxic prostate cancer cells 22RV1. Other researchers, such as Badiaga16\pard*, obtained an IC50 of 42.37 0.31 µg/mL by evaluating the antiproliferative capacity of T. macroptera, methanolic extract on prostate cancer PC3 cells using flow cytometry. Similarly, Tagne et al.17 showed that total alkaloid extracts from S. latifolius bark and roots were active on MCF-7 breast cancer cells with IC50 ranging from 12 to 39 µg/mL. Many reactive species are known to be implicated in the process of oxidative stress, inflammation and cancers18. Hydroxyl radical (OH•) which is one of the most powerful and the most prominent radical found in vivo is formed by the reaction of reactive oxygen radicals (O2) with hydrogen peroxide (H2O2) in the presence of Fe2+ or Cu+ as a catalyst. The antioxidant potential of the tested extract may then explain the potential of inhibiting cancer cell proliferation.
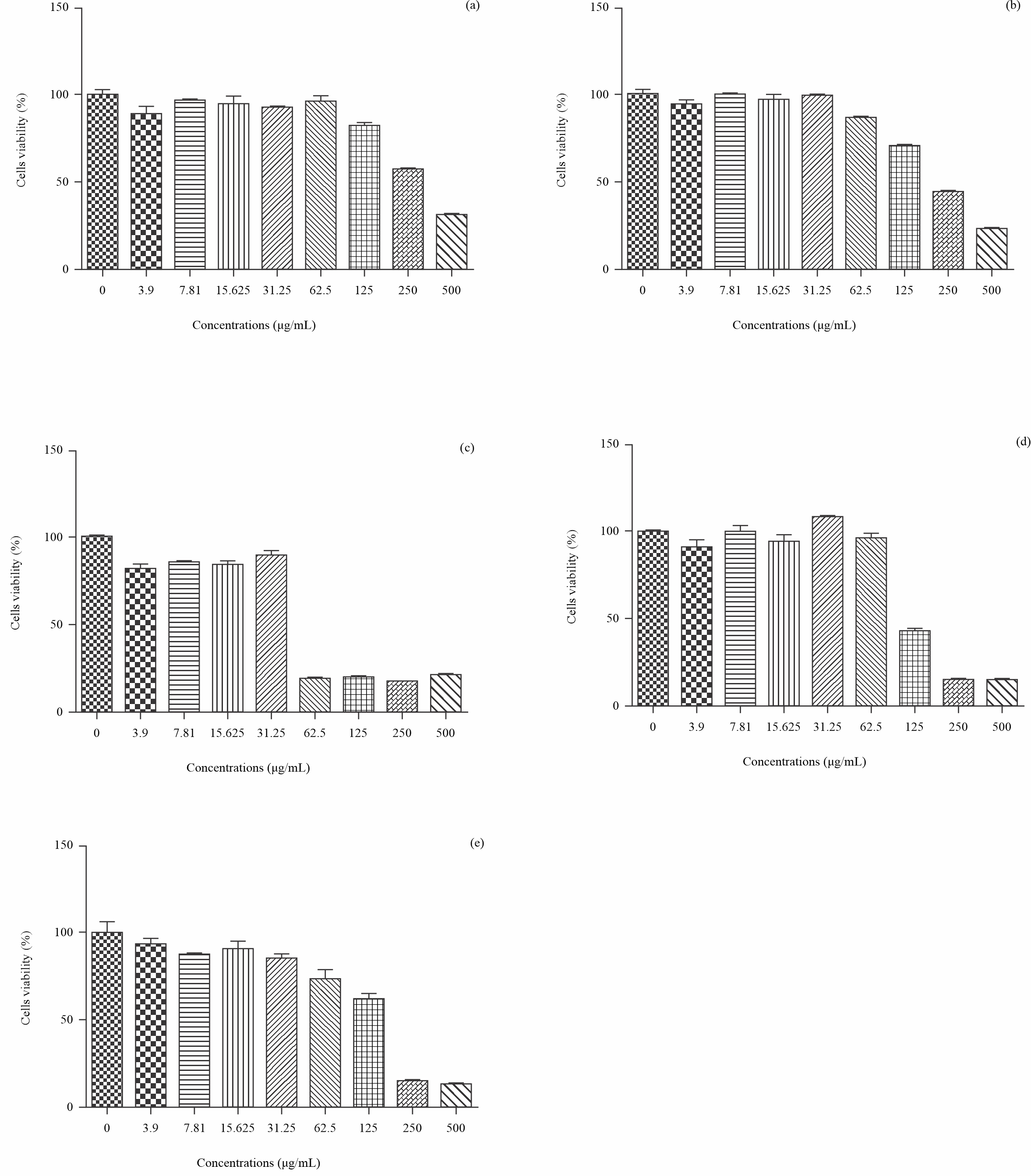 |
Fig. 4: Methanolic extracts toxicity on prostate cells 22RV1 (a): S. latifolius IC50: 359.1 µg/mL (tested concentrations range from 296.9 to 434.3), (b): T. macroptera IC50: 242.3 µg/mL (tested concentrations range from 210.3 to 279.1), (c): E. africana IC50: 48.34 µg/mL (tested concentrations range from 39.33 to 67.20), (d): Cassia sieberiana IC50: 48.34 µg/mL (tested concentrations range from 39.33 to 67.20), (e): C. planchonii IC50: 133.7 µg/mL (tested concentrations range from 111.2 to 160.8 |
CONCLUSION
This study was designed to collect information on the medicinal plants preconized by the traditional health practitioners of Bobo Dioulasso and evaluate the total phenolics and flavonoids content before assessing the antioxidant and anticancer potential of the selected plant. A total of 22 plants cited as being used in traditional treatment against malaria 5 had the highest citation frequencies. They presented significant content of total phenolics and total flavonoids. T. macroptera was the richness in phenolic content and was found to possess the best antioxidant potential while E. africana were the poorest in phenolic content, however, it showed the best cytotoxic activity. Cytotoxicity of the selected plant may be in relation toother class of metabolites different fromthose of polyphenols. The different results obtained on phenolic, flavonoid contents may justify the use of the tested plant in the treatment of malaria. The cytotoxicity of E. africana on prostate cancer cells 22RV1 will serve further in the investigation for research of other class of molecules and their isolation
ACKNOWLEDGMENT
The authors thank The International Atomic Energy Agency (IAEA) for its financial assistance to the projects No. BKF5021 awarded to Doctor Roland N-T Meda. The authors are also grateful to Doctor Hervé T Coulidiati for carrying out cytotoxicity assay (International Center for Genetic Engineering and Biotechnology, Cape Town, South Africa). The authors are grateful to Abdoul Kader Zangré –water and forests inspectors–for providing us access to the Traditional Healers Association of Bobo-Dioulasso.
REFERENCES
- Jamison, D.T., R.G. Feachem, W.M. Malegapuru, E.R. Bos and F.K. Baingana, F.K., Hofman, K.J., and Rogo, K.O., 2006. Disease and mortality in sub-saharan Africa. In: World Bank Book, World Bank (2nd Edn.), The World Bank United States.
- Kiemde, F., R. Spijker, P.F. Mens, H. Tinto, M. Boele and H.D.F.H. Schallig, 2016. Aetiologies of non-malaria febrile episodes in children under 5 years in sub-saharan Africa. Trop. Med. Int. Health, 21: 943-955.
- Bangou, M.J., K.B. Koama, T.H. Coulidiati, R.N.T. Meda, and A.M.E. Thiombiano et al., 2019. Phytochemical screening, antioxidant anf in vivo antiplasmodial activities of acacia gourmensis a. rich. (mimosaceae). Issues Bio. Sci. Pharm. Res., 7: 46-57.
- Ouédraogo, M., D.T. Kangoye, S. Samadoulougou, T. Rouamba, P. Donnen and F. Kirakoya-Samadoulougou, 2020. Malaria case fatality rate among children under five in Burkina Faso: an assessment of the spatiotemporal trends following the implementation of control programs. Int. J. Environ. Res. Public Health, Vol. 17.
- Coronado, L.M., C.T. Nadovich and C. Spadafora, 2014. Malarial hemozoin: from target to tool. Biochim. Biophys. Acta, 1840: 2032-2041.
- Valderramos, S.G. and D.A. Fidock, 2006. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci., 27: 594-601.
- Kannan, D., N. Yadav, S. Ahmad, P. Namdev, S. Bhattacharjee, B. Lochab and S. Singh, 2019. Pre-clinical study of iron oxide nanoparticles fortified artesunate for efficient targeting of malarial parasite. EBioMedicine.
- Lawal, B., O.K. Shittu, A.Y. Kabiru, A.A. Jigam, M.B. Umar, E.B. Berinyuy and B.U. Alozieuwa, 2015. Potential antimalarials from African natural products: A reviw. J. Intercult. Ethnopharmacol., 4: 318-343.
- Bernice, D., B.M. Jean, O. Paulin, O.Y. Hermann and G. Samson et al., 2020. Medicinal plants used in the treatment of hepatitis in bobodioulasso: studying the availability and analyzing the phytochemical properties of combretum micranthum g. don and entada Africana guill. et perr. Eur. Sci. J. ESJ, 16: 101-107.
- Rengasamy, G., A. Venkataraman, V.P. Veeraraghavan and M. Jainu, 2018. Cytotoxic and apoptotic potential of myristica fragrans houtt. (mace) extract on human oral epidermal carcinoma KB cell lines. Braz. J. Pharm. Sci., Vol. 54.
- Haidara, M., M. Haddad, A. Denou, G. Marti and S. Bourgeade-Delmas et al., 2018. In vivo validation of anti-malarial activity of crude extracts of terminalia macroptera, a malian medicinal plant. Malaria J., Vol. 17.
- Guigma, Y., P. Zerbo and J. Millogo-Rasolodimby, 2012. Use of spontaneous species in three contiguous villages in southern burkina faso. Tropicultura, 30: 230-235.
- Evenamede, K.S., K. Kpegba, O. Simalou, P. Boyode, A. Agbonon and M. Gbeassor, 2018. Comparative study of antioxidant activities of ethanolic extracts of leaves, bark and roots of Cassia sieberiana. Int. J. Bio. Chem. Sci., Vol. 11.
- Do, Q.D., A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji and Y.H. Ju, 2014. Effect of extraction solvent on total phenol content, total flavonoid content and antioxidant activity of Limnophila aromatica. J. Food Drug Anal., 22: 296-302.
- Bickii, J., G. Tchouya, J. Tchouankeu and E. Tsamo, 2007. Antimalarial activity in crude extracts of some Cameroonian medicinal plants. Afr. J. Trad. Compl. Alt. Med., 4: 107-111.
- Badiaga, M., 2011. Ethnobotanical, phytochemical and biological studies of Nauclea latifolia Smith, an African medicinal plant harvested in Mali. PhD., Université Blaise Pascal, Pages 136
- Tagne, R.S., B.P. Telefo, J.N. Nyemb, D.M. Yemele and S.N. Njina et al., 2014. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pacific J. Trop. Med., 7: S442-S447.
- Pharm-Huy, L.A., H. Hua and C. Pham-Huy, 2008. Free radicals antioxidants in disease and health. Biochemistry, 4: 89-96.
How to Cite this paper?
APA-7 Style
Bangou,
M.J., Thiombiano,
M.E., Habibou,
M., Ouedraogo,
S., Ouedraogo,
G.A. (2021). Inhibition of Prostate Cancer by Plant Extracts Used in the Treatment of Malaria in Burkina Faso. Asian Journal of Emerging Research, 3(1), 32-35. https://doi.org/10.3923/ajerpk.2021.32.35
ACS Style
Bangou,
M.J.; Thiombiano,
M.E.; Habibou,
M.; Ouedraogo,
S.; Ouedraogo,
G.A. Inhibition of Prostate Cancer by Plant Extracts Used in the Treatment of Malaria in Burkina Faso. Asian J. Emerg. Res 2021, 3, 32-35. https://doi.org/10.3923/ajerpk.2021.32.35
AMA Style
Bangou
MJ, Thiombiano
ME, Habibou
M, Ouedraogo
S, Ouedraogo
GA. Inhibition of Prostate Cancer by Plant Extracts Used in the Treatment of Malaria in Burkina Faso. Asian Journal of Emerging Research. 2021; 3(1): 32-35. https://doi.org/10.3923/ajerpk.2021.32.35
Chicago/Turabian Style
Bangou, Mindiédiba, Jean, M. Emmanuel Thiombiano, Mouniratou Habibou, Sibdou Ouedraogo, and Georges Anicet Ouedraogo.
2021. "Inhibition of Prostate Cancer by Plant Extracts Used in the Treatment of Malaria in Burkina Faso" Asian Journal of Emerging Research 3, no. 1: 32-35. https://doi.org/10.3923/ajerpk.2021.32.35

This work is licensed under a Creative Commons Attribution 4.0 International License.




